Workshop CH4203 Earth System Science
Fundamental constants: Planck’s constant, h = 6.626 * 10-34 Js, The speed of light, c = 3.00 * 108 ms-1, Avogadro’s constant, NA = 6.02 * 1023 mol-1, Ideal gas constant, R = 8.314 J mol-1 K-1. Assume T = 298 K and P = 1 Atm = 101,325 Nm-2 (unless the question states otherwise). Mixing ratio: ppbv = parts per billion by volume (10-9).
See also the “Some reaction kinetics you may have forgotten” summary on the kinetics revision work sheet covered at the start of the course.
Question 1
The bond dissociation energy of ozone (O3 + hv -> O2 + O) is 101 kJmol-1. Molecular oxygen and atomic oxygen both have low-lying electronically excited states with excitation energies of:
- Show that all four combinations of molecular and atomic products are energetically possible from ozone photolysis at 300 nm.
- Explain why only two of the four possible product combinations are actually observed from ozone photolysis at 300 nm. Which combinations are they and why?
Question 2
Electronically excited oxygen atoms are produced from the photolysis of ozone (1). They are then either quenched to form ground state O(3P) atoms by reaction with nitrogen (2) or oxygen (3), or react with water vapour to produce OH radicals (4):
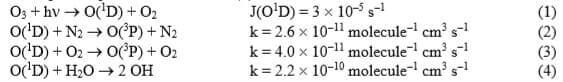
- What percentage of O(1D) atoms react to produce OH and what percentage are quenched?
- What is the production rate of OH?
- What is the concentration of O(1D) at steady state? Comment on the size of the answer.
[Assume that the atmosphere comprises 78% nitrogen, 21% oxygen and 1% water vapour, and assume [O3] = 30 ppbv].
Question 3
In the troposphere, the partitioning of NO and NO2 within the NOx family is determined (mainly) by the reactions:
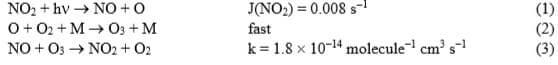
- What is the net chemical effect of reactions (1–3)?
- By applying photochemical steady-state arguments to the above reaction scheme, derive an equation for the ratio of NO2 and NO concentrations.
- Calculate the concentrations of NO and NO2 at photochemical steady-state for a total NOx concentration of 10 ppbv and an ozone concentration of 40 ppbv.

