1.2 current trends in copper production in kenya.
FINAL YEAR PROJECT PROPOSAL
PROJECT TITLE:
PROCESS OPTIMIZATION OF COPPER RECOVERY FROM LOW-GRADE ORES AND TAILINGS
Eng. BRIAN NZUKI AND Eng. BENSON NJUGUNA
A PROJECT PROPOSAL PRESENTED TO THE DEPARTMENT OF MINING, MATERIALS AND PETROLEUM ENGINEERING IN PARTIAL FULFILLMENT FOR THE AWARD OF BACHELORS DEGREE IN MINING AND MINERAL PROCESSING ENGINEERING
TABLE OF CONTENTS
Contents
1.2 Current trends in copper production in Kenya. 11
2.2 Methods of recovering copper 21
3.1.1 Equipment and Tools required 24
LIST OF FIGURES
Figure 4: Copper exploration map in Marsabit. . 10
Figure 5: Azurite copper ore. . 11
LIST OF TABLES
Table 4: First Semester Work Plan. 29
Table 5: Second Semester Work plan 29
DECLARATION
Name: Nderitu Eric Muchemi
Signature: ……………………. Date: ………………………………
SUPERVISOR APPROVAL
Name of Supervisor: Eng. Benson Njuguna
Signature: ……………………………
CHAPTER 1
1.0 INTRODUCTION
1.1 Research background
Figure 1: Kenyan map showing the general mineral distribution. (“Kenya Mining Cadastre Portal - Home: Cadastre Map,” 2021).
.
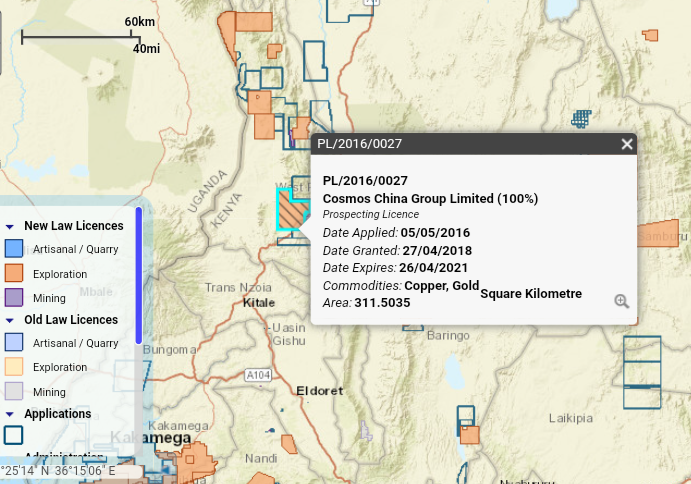
Figure 4: Copper exploration map in Marsabit. (“Kenya Mining Cadastre Portal - Home: Cadastre Map,” 2021).
1.2 Current trends in copper production in Kenya.
Figure 5: Azurite copper ore. (Drzymala et al., 2013).

1.3 Problem statement
Avoid wastage of copper from the low-grade ores.
Decrease the space occupied from the disposal of copper tailings.
1.4 Justification
1.5 Research objectives
1.5.1 Main objective
To optimise copper recovery from low-grade ores and tailings with unrecovered copper content >1% Cu concentration.
1.5.2 Specific objectives
To provide a future copper resource in Kenya to cater for the growing demand of copper.
CHAPTER 2
2.0 LITERATURE REVIEW
2.1 Major copper minerals
2.1.1 Malachite
2.1.2 Azurite
It is a minor ore of copper found largely in the oxidized portions of copper deposits. It is a secondary mineral formed by the action of carbonated water acting on copper-containing minerals or solutions such as CuSO4 or CuCl2 reacting limestone (Vink B., 1986). It is soluble in dilute acids, ammonia, and hot concentrated solutions of sodium hydrogen carbonate. It is slowly decomposed by boiling water, with CO2 effervescence. It is not extremely abundant but has various uses such as copper prospecting and mining as an indicator mineral. It helps geologists site sub-surface copper deposits. It can also be used as a piece of jewellery and ornamental stone for making beads, carvings, and ornaments. It can also be used to make blue pigments for painting once it has been ground into powder. It is also popular with mineral collectors due to its deep blue monoclinic crystals.
2.1.3 Chrysocolla
This copper mineral occurs as bright blue or green masses frequently coated with glossy quartz. It is of secondary origin and forms by oxidation in the oxidizing zones of copper ore bodies or a product of weathering. It occurs in form of massive rounded masses hence does not have a crystalline structure. Its chemical composition is unique as it has a microscopic mixture of copper hydroxide spertiniite (Cu(OH)2), amorphous silica and water proving to be a complex silicate mineral (Raghavan & Fuerstenau, 1977). It has a Mohr hardness of 2.5-3.5 and its fracture is sub-conchoidal to uneven. It is a minor copper ore hence used majorly as a source of copper metal. However, it can be used in jewellery though its usefulness depends largely on its intrinsic hardness.
2.1.4 Chalcopyrite
2.1.5 Native copper
2.1.6 Cuprite
2.1.7 Chalcocite
2.1.8 Covellite
This mineral can be classified in the copper sulphides category with the chemical formula of CuS. It generally occurs in the secondary enrichment zones (supergene) of copper sulphides deposits and forms in weathering environments restricted to hydrothermal conditions. It has a hexagonal crystalline structure with some of the copper ions being at the centre of sulphur tetrahedrons linked on their bases to form sheets (Tezuka et al., 2007). This indigo-blue mineral is found in limited abundance though being predominant in copper ores. Covellite has a range of uses one being that it is used in making batteries as it is a copper mineral. Others include being used in the making of jewellery due to its alluring blue hue and also in the mineral collection especially ones with high iridescence being prized.
2.2 Methods of recovering copper
2.2.1 Copper acid leaching
Copper acid leaching involves treating the low-grade copper ores and tailings; typically containing 1% Cu and >35% Fe along with silica and other elements (Mn, Al, Zn, Mb, Pb); with mainly a reducing acid and precipitating out the insoluble salt of the copper.
This process is aimed at efficient recovery of copper and removal of toxic metals from the tailings produced. Therefore with this leaching method, the factors affecting leaching efficiency will be investigated to ensure optimum conditions are set to conduct the experiment. These factors have been summarized in Table 3 below and they include:
Temperature
Consequently, oxide copper ore, mixed oxide-sulphide ores and low-grade copper sulphide ore, for economic reasons cannot be enriched by flotation techniques, however, can be processed by leaching methods (Banza, Gock, & Kongolo, 2002). Agents for leaching copper can be an acid (sulphuric acid), base (sodium hydroxide) and salt solutions (sodium hydrogen carbonate and sodium carbonate). Of all the listed agents, sulphuric acid is mostly preferred as it is a non-oxidizing acid in the optimum concentration applicable for the hydrometallurgy of copper. The basic copper carbonate minerals easily leached with the solution of sulphuric acid include azurite, malachite and chrysocolla (Lowson R., 1982). Oxidation reactions and leaching of copper minerals and pyrite from low-grade copper ores and mining tailings (waste, rock, overburden, oxidized portions of the ore body) proceed according to the following stoichiometric equations shown in the Table 2 below.
2.2.2 Copper recovery by solvent method
The extraction of copper from aqueous solution by the application of organic solvents such as LIX 64N or the Ashland Chemical products Kelex 100 and 120 proceeds chemically, according to the following equation:
| MINERALS | REACTIONS |
|---|---|
| Copper sulphides | |
|
Cu2S +1/2O2 + H2SO4→CuS + CuSO4 + H2O |
|
CuFeS2 + O2 + 2H2SO4→CuSO4 + FeSO4 + 2S +2H2O |
| CuFeS2 +2 Fe2(SO4)3→CuSO4 + FeSO4 + 2S + 2H2O | |
| Copper carbonates, oxides, and silicates | |
|
Cu2 (OH)2CO3+ 2H2SO4+ 2CuSO4 + 3H2O+ CO2 |
| Cu2O +1/2O2 + 2H2SO4→2CuSO4 +2H2O | |
| CuSiO3+2H2O + H2SO4→CuSO4 + SiO2+3H2O | |
| Cu3(OH)2(CO3)2+ 3H2SO4→ 3CuSO4 + 4H2O+ 2CO2 |
CHAPTER 3
3.0 METHODOLOGY
3.1 Data Collection
3.1.1 Equipment and Tools required
3.1.1.1 Fieldwork equipment and tools
These will include tools and equipment we shall use while in the field collecting the ore sample. They include:
Field books.
Lab work equipment and tools
Jaw crusher.
Ball mill.
3.1.2 Fieldwork procedure
The data collection process will begin with a visit to the various small scale mining sites in Kenya such as Kitui and Kirinyaga to collect malachite rock samples. We will collect low grade and middle-grade malachite rock samples, to be used for the lab work procedure. We will collect 50kg malachite samples and package them into bags for easier transportation. The malachite samples collected from the different locations will be blended to achieve a homogenous mixture.
3.2 Lab work procedure
This research will entail the use of locally available chemicals and low energy input requirements hence decreasing the expense of optimizing low-grade ores and flotation tailings.
The copper-rich slag to be used as a raw material will be sourced from the low-grade copper ores and tailings discarded from physical sorting and grading from the mines. The mineral composition and chemistry of the slag will be analysed using SEM-EDS (scanning electron microscope) for morphological analysis and X-ray fluorescence Spectroscopy for metal elemental analysis.
3.2.1 Leaching procedures
The leaching experiments will be conducted in a lab using a lab-scale reactor with a four-blade lab agitator. The temperature will be kept constant using a water bath system. Before conducting the experiments, the raw material will be homogenized using a sample divider to obtain a uniform and representative sample for analysis and leaching experiments. The particle sizes will be employed in the experimental work and the particle distribution analysis will be performed using 2mm-10mm sieves. Experiments will be conducted in such a way to ensure that the average leaching time is about 120 minutes. Sulphuric acid >99.5% assay is used for all the leaching experiments.
Table 3 below shows the experimental parameters to be used during this procedure and their respective levels.
Results analysis methods
This salt, copper carbonate will also be tested with the XRF scanner to confirm the increase of copper content extracted from the low-grade ore.
Thereafter it will be blended, if need be, with the medium grade copper ore to optimize its quality required for exportation.
WORKPLAN
| Month | May | June | July | August | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
| Literature review | |||||||||||||||||||
| Progress presentation | |||||||||||||||||||
| Proposal presentation | |||||||||||||||||||
BUDGET
| ITEM | COST (Ksh) |
|---|---|
| 4,000 | |
| 3,000 | |
| 3,000 | |
| 2,000 | |
|
3,000 |
|
3,000 |
| 2,000 | |
| 2,500 | |
| 21,500 |
CONCLUSION
References
Altundogan, H. S., Boyrazli, M., & Tumen, F. (2004). A study on the sulphuric acid leaching of copper converter slag in the presence of dichromate. Minerals Engineering, 17(3), 465–467.
Anand, S., Sarveswara Rao, K., & Jena, P. K. (1983). Pressure leaching of copper converter slag using dilute sulphuric acid for the extraction of cobalt, nickel and copper values. Hydrometallurgy, 10(3), 305–312.
Han, B., Altansukh, B., Haga, K., Stevanović, Z., Jonović, R., Avramović, L. … Shibayama, A. (2018). Development of copper recovery process from flotation tailings by a combined method of high‒pressure leaching‒solvent extraction. Journal of Hazardous Materials, 352, 192–203.
Heyes, G. W., & Trahar, W. J. (1979). Oxidation-Reduction effects in the flotation of chalcocite and cuprite. International Journal of Mineral Processing, 6(3), 229–252.
Northeya, S., Mohrb, S., Mudda, G.M., Wenga, Z., Giurcob, D. (2014) Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resources, Conservation and Recycling 83, 190– 201.
Pauling, L. & Brockway, L. (1932). The Crystal Structure of Chalcopyrite CuFeS2. Zeitschrift für Kristallographie - Crystalline Materials, 82(1-6), 188-194.
Tezuka, K., Sheets, W. C., Kurihara, R., Shan, Y. J., Imoto, H., Marks, T. J., & Poeppelmeier, K. R. (2007). Synthesis of covellite (CuS) from the elements. Solid-State Sciences, 9(1), 95–99.
Vink, B. W. (1986). Stability relations of malachite and azurite. Mineralogical Magazine, 50(355), 41–47.
