Ans btopic reaction products section difficulty level medium
1. Free radicals can be produced by:
A) use of high temperatures.B) irradiation with light.
2. As the term "peroxide" is used in Chapter 10, it can refer to which structure(s)? A) ROOR
B) ROOH
ΔHo=400 kJ/mol ΔHo=243 kJ/mol
A) +243 kJ / mol
B) -138 kJ / mol
C) +138 kJ / mol
D) -781 kJ / mol
E) +781 kJ / mol
Ans: B
ΔHo=423 kJ/mol ΔHo=193 kJ/mol
A) +616 kJ / mol
B) -101 kJ / mol
C) -173 kJ / mol
D) +57 kJ kJ / mol
E) -44 kJ / mol
Ans: E
ΔHo=421 kJ/mol ΔHo=243 kJ/mol
A) -121 kJ / mol
B) +121 kJ / mol
C) +243 kJ / mol
D) +664 kJ / mol
E) -785 kJ / mol
Ans: A
ΔHo=413 kJ/mol ΔHo=159 kJ/mol
A) +437 kJ / mol
B) -437 kJ / mol
C) -411 kJ / mol
D) +26 kJ / mol
E) -1581 kJ / mol
Ans: B
A) H–H ⎯⎯⎯⎯→ 2H·
B) H· + CH3–H ⎯⎯⎯⎯→ CH3–H + H· C) CH3· + CH3· ⎯⎯⎯⎯→ CH3–CH3
D) CH3· + CH3–H ⎯⎯⎯⎯→ CH3–H + CH3· E) Reactions (B) and (D)Ans: E
Ans: C
636
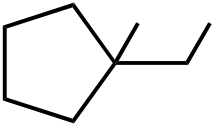
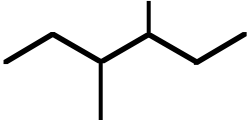
| 9. The ΔH° value is expected to be least for which indicated C-H bond of isopentane? | ||
|---|---|---|
| A) | H | |
CH3CCH2CH3
CH3
D) H
CH3
Ans: C
637
B) C) |
|||
|---|---|---|---|
| CH3CHCH2CH2 | |||
 |
|
||
|
|||
Ans: E
Topic: Reaction Products
Section: 10.3
Difficulty Level: Easy
12. Which of these molecules is not expected to arise as a product of the high temperature chlorination of methane?
C) is one that can be initiated by light.
D) involves a series of steps, each of which generates a reactive intermediate that brings about the next step.
A) Cl–Cl ⎯⎯⎯⎯→ 2Cl·
B) Cl· + CH4⎯⎯⎯⎯→ CH3· + H–Cl
C) CH3· + CH3· ⎯⎯⎯⎯→ CH3–CH3
D) CH3· + Cl–Cl ⎯⎯⎯⎯→ CH3Cl + Cl·
E) More than one of the above
Ans: C
639
H–Cl, ΔH° = 432 kJ mol -1
This mechanism is unlikely because:
A) The overall ΔH° is highly endothermic.
16. Which of the reactions listed below would be exothermic?
A) CH3–CH3⎯⎯⎯⎯→ 2CH3·
B) CH3· + CH4⎯⎯⎯⎯→ CH4 + CH3·
C) 2(CH3)2CH· ⎯⎯⎯⎯→ (CH3)2CH–CH(CH3)2 D) H· + (CH3)3CH ⎯⎯⎯⎯→ (CH3)3CH + H· E) None of the above
Ans: C
| B) | Cl |  |
H | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C) | Cl |
|
H | ||||||||
| δ - | H | ||||||||||
| Cl | |||||||||||
| D) | Cl | H | |||||||||
| Cl |
C |
H | |||||||||
| E) | + | C | H | ||||||||
| δ |  |
|
H | ||||||||
| Cl | |||||||||||
|
H | ||||||||||
18. For which of the following reactions would the transition state most resemble the products? The following bond dissociation energies may be useful.
(CH3)2CH–H CH3CH2CH2–H H–F (413 kJ mol-1) (423 kJ mol-1) (570 kJ mol-1) H–Cl H–Br
(432 kJ mol-1) (366 kJ mol-1)
A)
A) CH2ClCHCl2
B) CH3CHCl2
C) CH3CH2Cl
D) ClCH2CH2Cl
E) All of these
Ans: E
Topic: Reaction Products (Isomers)
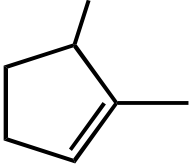
Section: 10.3, 10.5
Difficulty Level: Medium
21. The reaction of 2-methylbutane with chlorine would yield how many monochloro derivatives? (include stereoisomers)
A) 2
B) 3
C) 4
D) 5
E) 6
Ans: E
Topic: Reaction Products (Isomers)
Section: 10.3, 10.5
Difficulty Level: Medium
A) A new free radical is formed.
B) The process is endothermic.
643
Topic: Activation Energies
Section: 10.5B
Difficulty Level: Easy
25. Which of the following reactions would have an activation energy equal to zero?
Ans: C
644
28. Which of the following reactions would have the smallest energy of activation?
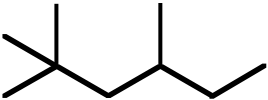
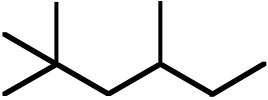
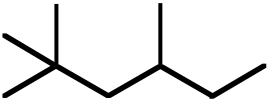
Topic: Activation Energies
+ Br

. + HBr
C)
. + HBr
E)
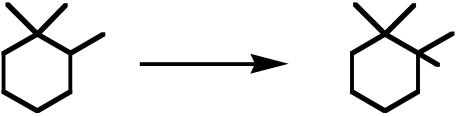
30. An example of a reaction having an Eact = 0 would be: A) Br· + Br–Br ⎯⎯⎯⎯→ Br–Br + Br·
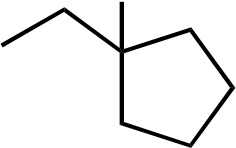
B) F· + CH4⎯⎯⎯⎯→ H–F + CH3·
C) CH3· + CH3CH3⎯⎯⎯⎯→ CH4 + CH3CH2· D) Br· + H–Br ⎯⎯⎯⎯→ H–Br + Br·
E) CH3· + CH3· ⎯⎯⎯⎯→ CH3–CH3
Ans: E
646
| + | + | HCl | + |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||
| CH3CHCH2 |  |
|||||||||||||
| + | Cl | |||||||||||||
| CH3 | CH3 | HCl | ||||||||||||
CH3CCH3 |
||||||||||||||
| + | Cl | |||||||||||||
| CH3 | CH3 | |||||||||||||
| CH3 | C | CH3 | + | Cl |  |
CH3 | CH2 | |||||||
|
|
|||||||||||||
ΔH° (kJ mol-1) A) CH3· + CH3· ⎯⎯⎯⎯→ CH3CH3 -378
B) CH3· + Br· ⎯⎯⎯⎯→ CH3Br -130
C) CH4 + I· ⎯⎯⎯⎯→ CH3· + HI +142
D) CH4 + Br· ⎯⎯⎯⎯→ CH3· + HBr +104
E) CH4 + Cl· ⎯⎯⎯⎯→ CH3· + HCl +8
Ans: C
647
Topic: Activation Energies
Section: 10.2, 10.5B
Difficulty Level: Medium
predict which of the following reactions would have the highest energy of activation.
A) CH4 + F· ⎯⎯⎯⎯→ CH3· + HF
B) CH4 + Cl· ⎯⎯⎯⎯→ CH3· + HCl
C) CH4 + Br· ⎯⎯⎯⎯→ CH3· + HBr
D) CH4 + I· ⎯⎯⎯⎯→ CH3· + HI
E) ΔH° values are important, but not sufficient for this prediction Ans: D
Topic: Mechanisms
Section: 10.5C
Difficulty Level: Medium
36. Which of the following statements is true when used to compare the reaction of fluorine with 2-methylhexane and the reaction of bromine with 2-methylhexane?
E) More than one of the above statements is true.
Ans: C
38. When an alkane in which all hydrogen atoms are not equivalent is monosubstituted, use of this halogen produces a ratio of isomers which is essentially statistical, i.e., dependent only on the number of each type of hydrogen.
A) F2
B) Cl2
C) Br2
D) I2
E) All of the above
Ans: A
heat or light
D) The signal for the methyl protons would be a doublet.
E) The signal for the methyl protons would integrate for only 2 hydrogens.
A) Ethane
B) Propane
C) Butane
D) Isobutane
E) Pentane
Ans: D
Topic: Reaction Products
Section: 6.18, 8.16, 10.6
Difficulty Level: Hard
42. Select the structure of the major product formed in the following reaction.
CH2Br |
Br2 |
|
|||
|---|---|---|---|---|---|
CH3
|
Br | ||||
| I | II | IV | |||
|
|||||
A) I
B) II
C) III
D) IV
E) V
Ans: B
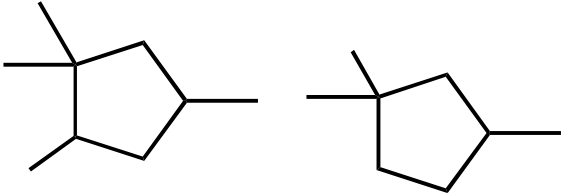
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Easy
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Easy
| 45. | Mono-bromination of the following alkane, |  |
|
|---|
| I | II | III | IV | V |
|---|
B) II
C) III
D) IV
E) V
Ans: A
653
Br
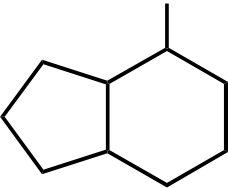
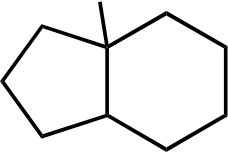
Br
Br Br Br
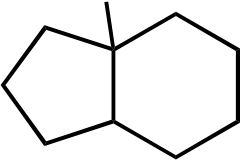
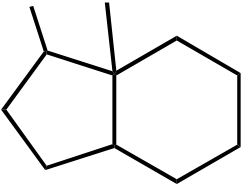
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Easy
48.
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Easy
be?
| Br | Br |
|
|||||
|---|---|---|---|---|---|---|---|
| III | IV | ||||||
| I | II | V | |||||
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
50. Which hydrogen would be abstracted first when mono-brominating with Br2 and light?
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
51. Which hydrogen would be abstracted first when mono-brominating with Br2 and light? Hc Hc
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
52. Which hydrogen would be abstracted first when mono-brominating with Br2 and light?
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
53. Which hydrogen would be abstracted first when mono-brominating with Br2 and light?
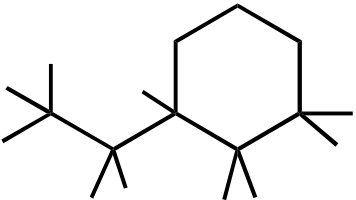
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
| He | Hb Hb | ||
|---|---|---|---|
| He | |||
|
A) Ha
B) Hb
C) Hc
D) Hd
E) He
Ans: D
56. Free radical fluorination of an alkane is not typically conducted because of fluorine’s high reactivity due to
A) the low energy of activation for the chain-propagating steps.B) the large negative overall ΔHo for the reaction.
Topic: Reaction Products
Section: 10.6A
Difficulty Level: Medium
57. Free radical iodination of an alkane is not feasible because
A) the iodine-iodine bond is relatively weak compared to the other halogens. B) the overall ΔHo for the reaction is favorable.
B) The transition state of the rate determining step is product-like.
C) The major product formed from this reaction is 1-bromo-2-methylpropane.
B) II
C) III
D) IV
E) V
Ans: C
 |
 |
|
|
 |
|---|---|---|---|---|
| 2. CH3CH2OH, heat | ||||
| I | II | III |
|
V |
Topic: Reaction Products
Section: 6.18, 10.6A
Difficulty Level: Medium
62. What would be the major product of the following reaction sequence?
| OH | Ot-Bu | |||
|---|---|---|---|---|
 |
||||
| I | II | III | IV | V |
|
||||
B) II
C) III
D) IV
E) V
Ans: C
Topic: Reaction Products
Section: 6.18, 10.6A
Difficulty Level: Medium
 |
 |
||||
|---|---|---|---|---|---|
|
|||||
| O | |||||
| I | II | III | IV | V | |
65. For which reaction would the transition state be most product-like?
 |
|
SCH3 | |||
|---|---|---|---|---|---|
| DMF | |||||
|
|||||
| I | II | V | |||
| III | IV | ||||
1. Br2, hν
Topic: Reaction Products
Section: 6.18, 10.6A
Difficulty Level: Hard
 |
OEt | ||||
|---|---|---|---|---|---|
 |
|||||
|
|||||
|
 |
||||
| I | II | V | |||
| III | IV | ||||
Br
|
 |
|||
|---|---|---|---|---|
|
||||
 Br Br |
||||
| Br | ||||
| I | II | III | IV | |
| V | ||||
A) I
B) II
C) III
D) IV
E) V
Ans: C
665
72. What is the product for the following three-step reaction sequence?
|
 |
|||
|---|---|---|---|---|
|
||||
O
|
||||
| I | II | III | IV | V |
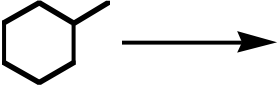
Topic: Multistep Reactions
Section: 6.18, 8.7, 10.6A
Difficulty Level: Hard
| Br2 | A | B |
|
||||
|---|---|---|---|---|---|---|---|
| hν | |||||||
| heat | |||||||
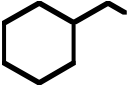 OH OH |
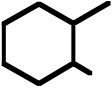 OH OH |
OH |
|||||
| I | II | III | IV | ||||
75. What is the product for the following three-step reaction sequence?
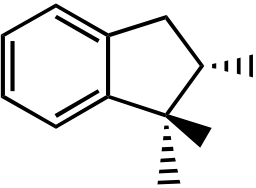
Topic: Synthesis
Section: 6.18, 8.12, 10.6A
Difficulty Level: Hard
|
V | Br | Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Br |
Br |
|||||||
| I | II | III | IV | ||||||
77. What is the product for the following three-step reaction sequence?
|
|
|
OH |
|
||||
|---|---|---|---|---|---|---|---|---|
 |
||||||||
| I | II | III | IV | V |
Topic: Synthesis
Section: 6.18, 8.16, 10.6A
Difficulty Level: Hard
78. What is the product for the following three-step reaction sequence?
670
| OH |
|
 |
OH | |||
|---|---|---|---|---|---|---|
 |
||||||
| OH | ||||||
| CO2H | ||||||
| CO2H | ||||||
| I | II | III | IV | V | ||
A) I
B) II
C) III
D) IV
E) V
Ans: D
Topic: Synthesis
Section: 6.18, 8.17, 10.6A
Difficulty Level: Hard
80. What is the product for the following three-step reaction
sequence?
CHO
|
 |
OH | OH |
||
|---|---|---|---|---|---|
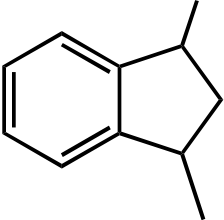 |
|||||
| O | |||||
| I | II | III | IV | V |
|
OH |
OH | 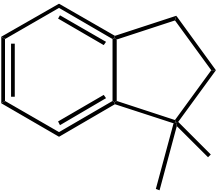 OCH3 OCH3 |
||
|---|---|---|---|---|---|
| O | |||||
| I | II | III | IV | V |
A) I
B) II
C) III
D) IV
E) V
Ans: A
Topic: Free Radicals
Section: 10.7
Difficulty Level: Easy
672
Topic: Free Radicals
Section: 10.7
Difficulty Level: Easy
Topic: Reaction Products (Isomers)
Section: 10.3, 10.5, 10.8
Difficulty Level: Medium
85. The free radical chlorination of pentane produces this number of monochloro compounds, including stereoisomers.
Topic: Reaction Products (Isomers)
Section: 10.3, 10.5, 10.8
Difficulty Level: Medium
87. Free radical chlorination of hexane produces this number of monochloro derivatives (including stereoisomers):
A) 3
B) 4
C) 5
D) 7
E) 8
Ans: C
89. What is the total number of trichloropropanes which can be produced by free radical chlorination of propane? Include all stereoisomers.
A) 4
B) 5
C) 6
D) 7
E) 8
Ans: C
E) one racemic mixture.
Ans: B
Topic: Reaction Products (Isomers)
Section: 10.3, 10.5, 10.8
Difficulty Level: Hard
92. The free radical chlorination of 3-chloropentane forms a mixture of dichloropentanes which, on precise fractional distillation, affords these fractions:
A) 4 fractions, none optically active
B) 4 fractions, 2 optically active
C) 7 fractions, 4 optically active
D) 7 fractions, 6 optically active
E) 7 fractions, all optically active
Ans: A
Topic: Reaction Products
Section: 10.9
Difficulty Level: Easy
94. What would be the major product of the following reaction?
 |
|
? | |||||
|---|---|---|---|---|---|---|---|
CH3
|
|||||||
| I | II | Br | V | ||||
|
|
||||||
96. The reaction of 1-butene with HBr in the presence of peroxides yields 1-bromobutane.
The mechanism for the reaction involves:
A) attack on the alkene by a Br+ ion.Ans: C
Topic: Synthesis
Section: 10.9
Difficulty Level: Medium
| A) | CHCH2CH2CH2CH3 | |||||
|---|---|---|---|---|---|---|
| B) | ||||||
|
||||||
|
CHCH2CH2CH2CH3 | |||||
| C) | ||||||
| + HBr | ||||||
| CH3CH2 | ||||||
| D) | ||||||
| CH3CH2 |
|
|
||||
Topic: Reaction Products
Section: 10.9
Difficulty Level: Medium
98. Which of the following combinations of reactants can provide a demonstrable example of anti-Markovnikov addition?
99. 2-Methyl-2-butene reacts with HBr in the presence of peroxide to give (chiefly): A) (CH3)2CHCH2CH2Br
B) (CH3)2CHCHCH3
| E) |
|---|
Topic: Reaction Products
Section: 6.5, 10.9
Difficulty Level: Medium
100. What would be the major product of the following reaction sequence?
Topic: Reaction Products
Section: 6.5, 10.9
Difficulty Level: Medium
101. What would be the major product of the following reaction sequence?
 |
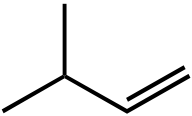 |
1. HBr, ROOR, hν | |||
|---|---|---|---|---|---|
|
|||||
| CN | |||||
| I | II | III | IV | V | |
1. HBr, ROOR, hν
2. CH3CH2OH
B) II
C) III
D) IV
E) V
Ans: A
680
| Ot-Bu | Ot-Bu | |||
|---|---|---|---|---|
 |
||||
|
||||
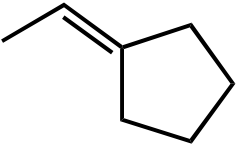 |
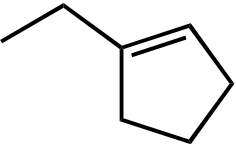 |
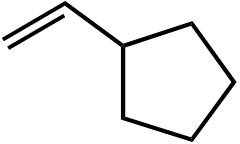 |
||
 |
||||
| I | II | III | IV | V |
B) II
C) III
D) IV
E) V
Ans: B
681
 |
|
|---|
3. HBr, ROOR, hν
A) I
B) II
C) III
D) IV
E) V
Ans: E
 |
|
|---|
3. HBr, ROOR, hν
A) I
B) II
C) III
D) IV
E) V
Ans: B
1. HBr, ROOR, hν
2. t-BuOK, t-BuOH, heat
 |
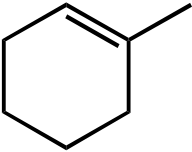 |
OEt | 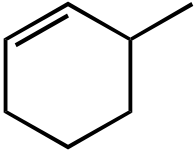 |
OH | |
|---|---|---|---|---|---|
 |
|||||
| I | II | III | IV | V | |
Topic: Reaction Products
Section: 6.18, 10.9
Difficulty Level: Medium
108. What would be the major product of the following reaction sequence?
 Br Br |
|
 |
||
|---|---|---|---|---|
| I | II | III | IV | V |
|
B) II
C) III
D) IV
E) V
Ans: C
110. What would be the major product of the following reaction sequence?
| Br | Cl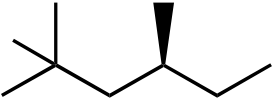 |
|
 |
||
|---|---|---|---|---|---|
| Br | |||||
| Br | Br | ||||
| + | |||||
diastereomer
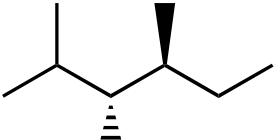
111. What would be the major product of the following reaction sequence?
| Br | Cl |
|
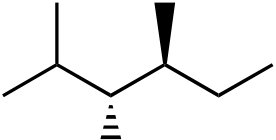 |
||
|---|---|---|---|---|---|
| Br | |||||
| Br | Br | ||||
| + | |||||
diastereomer
 |
 |
OCH3 | |||
|---|---|---|---|---|---|
| OH |  |
||||
| I | II | V | |||
| III | IV | ||||
B) II
C) III
D) IV
E) V
Ans: A
685
114. Which would be the best way to carry out the following synthesis? ?
(CH3)3COH
(CH3)2CHCH2Br
| NC |  |
|
 |
|||
|---|---|---|---|---|---|---|
| NC | ||||||
 |
 |
|||||
|
II | III | IV | V | ||
A) I
B) II
C) III
D) IV
E) V
Ans: B
117. What is the major product obtained from the following reaction sequence?
HBr peroxides A
t-BuOK t-BuOH B
OH
688
|
|
|||
|---|---|---|---|---|
 |
||||
| I | II | III | IV | V |
NaSH D hν t-BuOH peroxides
heat
SH
SH
SH
Topic: Multistep Reactions
Section: 6.5, 6.18, 10.6A, 10.9
Difficulty Level: Hard
120. What is the major product obtained from the following reaction sequence?
121. If propene polymerization is initiated by the use of diacyl peroxide, this is an intermediate species formed early in the process.
A) |
CH3
|
||||
|---|---|---|---|---|---|
| RCH2CHCH2CH | |||||
|
|||||
| RCHCH2CHCH2 | |||||
| RCHCH2CH2CH | |||||
|
|||||
|
|||||
| RCCH2CH | |||||
| CH3 | |||||
690
Topic: Miscellaneous
Section: 10.11D
Difficulty Level: Medium
123. Intermediates possessing unpaired electrons are called ______________. Ans: radicals or free radicals
Topic: General
Section: 10.1
Difficulty Level: Easy
691
Topic: General
Section: 10.2B
Difficulty Level: Medium
Topic: General
Section: 10.4
Difficulty Level: Easy
128. In a chain-initiating step, radicals are _____________. Ans: created or formed
692
Ans: The energy of activation for the first propagation step in the free radical
mechanism for halogenation of alkanes determines the overall reactivity pattern for the various halogens. In contrast to the reaction with the other halogens, hydrogen atom abstraction from an alkane by an iodine atom has a very high energy of activation- since this is the first propagation step, the overall process is affected as well and iodination does not occur to any appreciable extent.I . + R-H à H-I + R .
133. Briefly, but clearly, explain the following observation:
When 2-methylbutane reacts with Cl2/hν, the monochlorinated products consist of four constitutional isomers in significant yields. However, when the same alkane is allowed to react with Br2/ hν, there is only one major monobromination product.Ans: The final product distribution is a consequence of the relative ease of hydrogen atom abstraction from primary, secondary and tertiary positions. Chlorine is highly reactive and therefore there is not much differentiation between the different kinds of hydrogen atoms- as a result, all the possible free radicals are formed, leading to the formation of all possible constitutional isomers. Bromine is less reactive and more selective than chlorine in its reaction with alkanes; this results in the selective abstraction of the tertiary hydrogen atom at C2, to give the most stable radical intermediate, a tertiary radical, leading to the selective formation of only one major monobromination product.
 |
Cl | Cl |
|---|
694
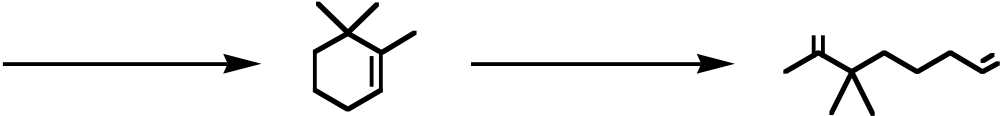
| Ans: |  |
+ |
|
 |
|
|||
|---|---|---|---|---|---|---|---|---|
|
heat | BH3, THF |
||||||
|
|
+ | ||||||
136. Draw bond-line formulas of all monochloro derivatives that might be formed when 2,3-dimethylbutane is allowed to react with Cl2 under UV irradiation. For each structure, indicate, with an asterisk, any stereocenters that might be present.
| Ans: |  |
Cl2, hν |
Cl | |||
|---|---|---|---|---|---|---|
| Cl | * | + | ||||
Topic: Reaction Products (Isomers)
Section: 10.6, 10.8
Difficulty Level: Medium
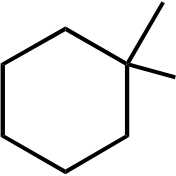
137. Draw bond-line formulas of all monochloro derivatives that might be formed when 1,1-dimethylcyclobutane is allowed to react with Cl2 under UV irradiation. For each structure, indicate, with an asterisk, any stereocenters that might be present.
1,1-dimethylcyclobutane Topic: Reaction Products (Isomers) Section:
10.6, 10.8
Difficulty Level: Medium
| Ans: | Cl2 |
Cl | + | + | Cl |
|||
|---|---|---|---|---|---|---|---|---|
Ans: Cl
140. Draw bond-line formulas of all dichloro derivatives that might be formed when 1-chloro-2,2,3,3,-tetramethylpentane is allowed to react with Cl2 under UV irradiation. For each structure, indicate, with an asterisk, any stereocenters that might be present.
| Ans: | Cl | Cl | + | Cl |
|
+ | Cl | Cl | + | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl | + | Cl | ||||||||||||||
| tetramethylpentane | ||||||||||||||||
| * | ||||||||||||||||
dichloro derivatives
Topic: Reaction Products (Isomers)
Section: 10.6, 10.8
Difficulty Level: Medium
monochloro derivatives
Ans: radical or free-radical; anti-Markovnikov
Topic: General
Section: 10.9
Difficulty Level: Medium
Ans: The addition of HBr to 2-methylpropene takes place via the formation of the most stable free radical intermediate, by addition of a bromine atom at C1. Two such radicals can couple together via a termination step to afford 1,4-dibromo-2,2,3,3-tetramethylbutane as a minor product. Analogous coupling between this radical and a bromine radical leads to the formation of 1,2-dibromo-2-methylpropane as another minor product.
|
Br | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||
| Br | + |  |
|
 |
Br |  |
|||||
| Br+ | Br | ||||||||||
termination steps: radical coupling
698
heat
|
|---|
iv) NaCN
Topic: General
Section: 10.10
Difficulty Level: Easy
147. Macromolecules made up of many repeating subunits are called ____________. Ans: polymers
Topic: General
Section: 10.11D
Difficulty Level: Easy
149. Chlorofluorocarbons, or freons, diffuse into the upper atmosphere. There, ultraviolet light initiates a radical chain reaction that has been shown to cause extreme damage to _______________.







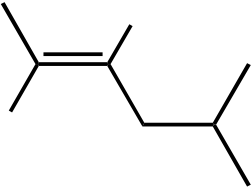

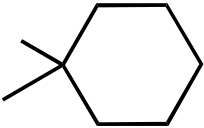


 OH
OH
 Cl
Cl
 CHO
CHO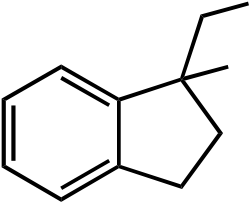
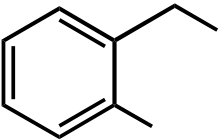 CHO
CHO
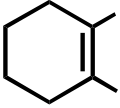
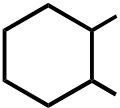 OR
OR




 BH2 H
BH2 H