Data analysts and data management systems charged costs
*A complete list of SOLIDARITY Trial investigators is
provided in the Supplementary Appendix.
Hongchao Pan, Ph.D., Richard Peto, F.R.S., Quarraisha Abdool Karim, Ph.D., Marissa Alejandria M.D., M.Sc., Ana Maria Henao-Restrepo, M.D., M.Sc., César Hernández García M.D., Ph.D., Marie-Paule Kieny Ph.D., Reza Malekzadeh M.D., Srinivas Murthy M.D. C.M., Marie-Pierre Preziosi M.D., Ph.D., Srinath Reddy M.D., D.M., Mirta Roses Periago M. D., Vasee Sathiyamoorthy B.M.B.Ch., Ph.D., John-Arne Røttingen M.D., Ph.D., and Soumya Swaminathan M.D. , as the members of the Writing Committee, assume responsibility for the content and integrity of this article.
Writing committee affiliations: Nuffield Department of Population Health, University of Oxford, Oxford (H.P. and R.P.); Centre for the AIDS Programme of Research In South Africa (CAPRISA), Durban, South Africa (Q.A.K.); National Institutes of Health, Manila, University of the Philippines, Manila (M.M.A.); Agency of Medicine and Medical Devices, Madrid, Spain (C.H.G); Institut National de la Santé Et de la Recherche Médicale (INSERM), Paris, France (M.P.K.); Ministry of Health and Medical Education, Tehran, Iran (R.M.); University of British Columbia, Vancouver, Canada (S.M); Public Health Foundation of India, New Delhi, India (K.S.R.); National Academy of Sciences of Buenos Aires, Buenos Aires, Argentina (M.R.P.); Research Council of Norway, Oslo, Norway (J-A.R.); World Health Organization, Geneva, Switzerland (A-M.H-R., M-P.P., V.S.M., S.S.).
Address correspondence and reprint requests to WHO R&D Blueprint at 20 Avenue Appia, 1211 Geneva, Switzerland or
METHODS
Study drugs were Remdesivir, Hydroxychloroquine, Lopinavir (fixed-dose
combination with Ritonavir) and Interferon-�1a (mainly subcutaneous;
initially with Lopinavir, later not). COVID-19 inpatients were
randomized equally between whichever study drugs were locally available
and open control (up to 5 options: 4 active and local standard-of-care).
The intent-to-treat primary analyses are of in-hospital mortality in the
4 pairwise comparisons of each study drug vs its controls (concurrently
allocated the same management without that drug, despite availability).
Kaplan-Meier 28-day risks are unstratified; log-rank death rate ratios
(RRs) are stratified for age and ventilation at entry.
RESULTS
In 405 hospitals in 30 countries 11,266 adults were randomized, with
2750 allocated Remdesivir, 954 Hydroxychloroquine, 1411 Lopinavir, 651
Interferon plus Lopinavir, 1412 only Interferon, and 4088 no study drug.
Compliance was 94-96% midway through treatment, with 2-6% crossover.
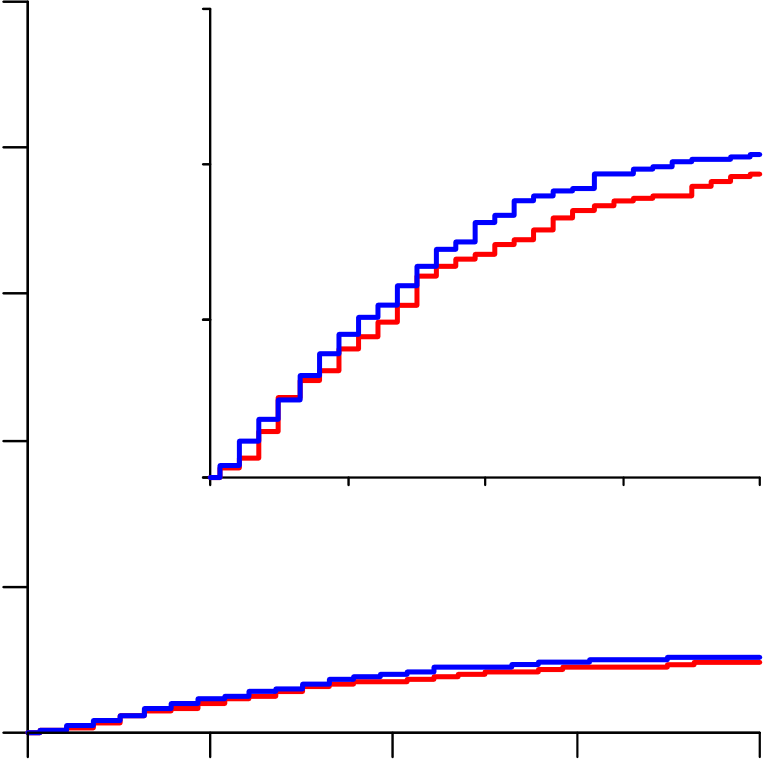
1253 deaths were reported (at median day 8, IQR 4-14). Kaplan-Meier
28-day mortality was 12% (39% if already ventilated at randomization,
10% otherwise). Death rate ratios (with 95% CIs and numbers
dead/randomized, each drug vs its control) were: Remdesivir RR=0.95
(0.81-1.11, p=0.50; 301/2743 active vs 303/2708 control),
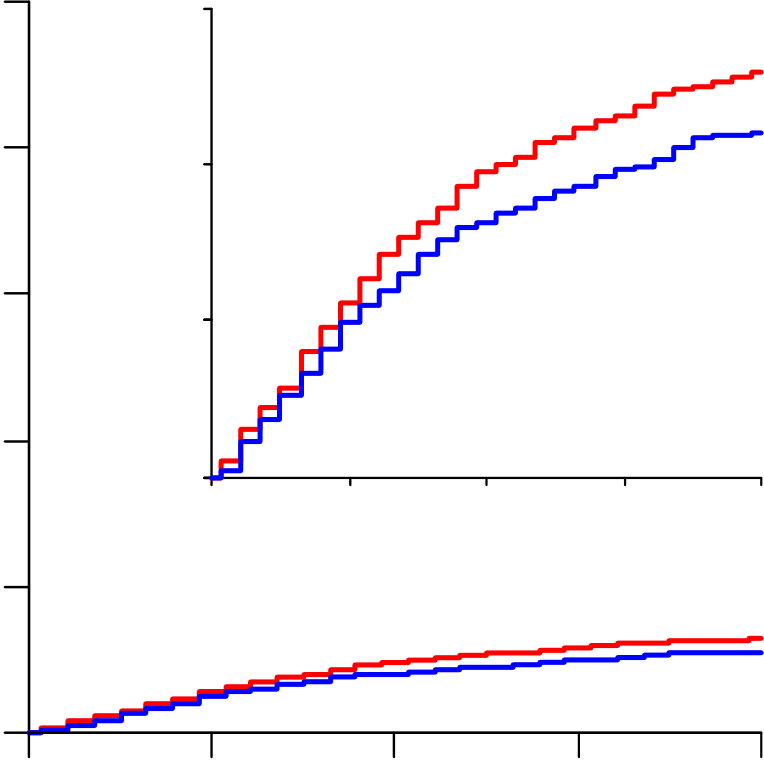
Hydroxychloroquine RR=1.19 (0.89-1.59, p=0.23; 104/947 vs 84/906),
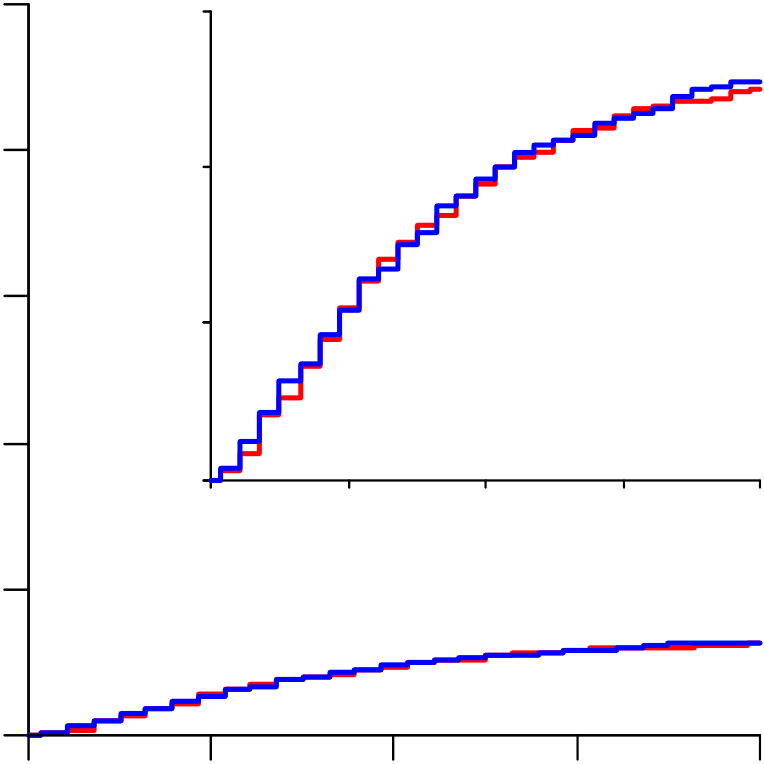
Lopinavir RR=1.00 (0.79-1.25, p=0.97; 148/1399 vs 146/1372) and
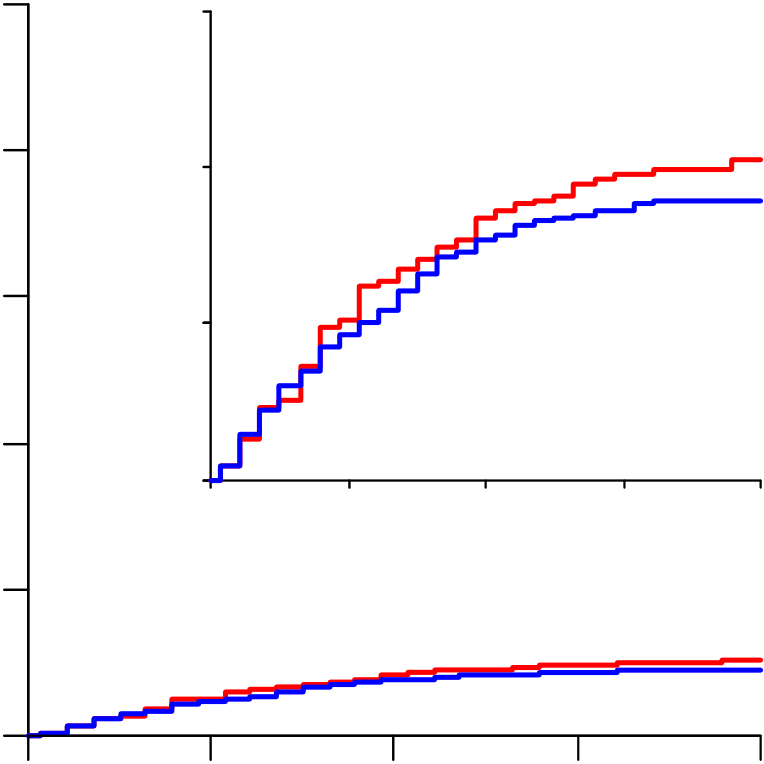
Interferon RR=1.16 (0.96-1.39, p=0.11; 243/2050 vs 216/2050). No study
drug definitely reduced mortality (in unventilated patients or any other
subgroup of entry characteristics), initiation of ventilation or
hospitalisation duration.
METHODS
The protocol3 was designed to involve hundreds of potentially over-stressed hospitals in dozens of countries. Hence, no form-filling was required, and trial procedures were minimal but rigorous. Online randomization of consented patients (via a cloud-based GCP-compliant clinical data management system) took just a few minutes, as did online reporting of death in hospital or discharge alive (plus brief details of respiratory support in hospital and use of study drugs and certain non-study drugs). No other reporting was required unless doctors suspected an unexpected serious adverse reaction (SUSAR). National and global monitors resolved queries and checked progress and data completeness. Eligible patients were age ≥18 years, hospitalized with a diagnosis of COVID-19, not known to have received any study drug, without anticipated transfer elsewhere within 72 hours, and, in the physician�s view, with no contra-indication to any study drug. Participants were randomized in equal proportions between control and whichever other study drugs were locally available (up to 5 options: these drugs, and local standard-of-care). Placebos were not used. Study drugs were Remdesivir, Hydroxychloroquine, Lopinavir-Ritonavir and Interferon (given with Lopinavir, until July 4). Hydroxychloroquine and Lopinavir were discontinued for futility on June 18 and July 4, 2020, respectively; Interferon is ceasing on October 16.
Interferon (mainly subcutaneous): Three doses over six days of 44µg subcutaneous Interferon-ß1a; where intravenous interferon was available, patients on high-flow oxygen, ventilators or ECMO were instead to be given 10µg intravenously once daily for six days.
ENDPOINTS
The protocol-specified primary objective was to assess effects on
in-hospital mortality (ie, mortality during the original episode of
hospitalization; follow-up ceased at discharge) not only in all patients
but also in those with moderate COVID and in those with severe COVID
(subsequently defined as ventilated when randomized).
5
All analyses relate mortality to allocated treatment (ie, they are
intent-to-treat analyses). The overall mortality analyses were of all
randomised patients (drug vs its control), and the only
protocol-specified subgroup analyses are those considering separately
patients with moderate and with severe COVID (ie, already ventilated;
the type of ventilation was not recorded at study entry.)
Unstratified Kaplan-Meier methods plot 28-day risk. Death rate ratios (RRs) and p-values are from log-rank analyses, stratified for 3x2=6 strata of age and ventilation at entry. If the stratified log-rank Observed minus Expected number of deaths is O-E with variance V, logeRR is calculated as (O-E)/V with variance 1/V and a Normal distribution.8 The few currently uncertain death times were taken as day 7. Analyses censored patients with outcome not yet reported at day 0, and censored the few inter-hospital transfers at transfer. They did not censor patients discharged alive, as analyses were of mortality during the initial hospitalisation. Forest plots (with 95% CIs only for overall results, otherwise 99% CIs) and chi-squared statistics (sum of [O-E]2/V, with no p-value given) help interpret any apparent heterogeneity of treatment RRs between subgroups. Analyses used SASv9.4 and Rv4.02.
RESULTS
From March 22 to October 4, 2020, 11,330 patients were entered from 405 hospitals in 30 countries in all 6 WHO regions. Of these, 64 (0.6%) had no, or uncertain, consent to follow-up, leaving 11,266 for intent-to-treat analyses: 2750 allocated Remdesivir, 954 Hydroxychloroquine, 1411 only Lopinavir-ritonavir, 2063 Interferon, and 4088 no study drug (Figure 1; reporting is 97% complete for those entered >1 month earlier, and 99.7% complete for those entered >3 months earlier). All 3 patients with COVID refuted are included, and survived.
medRxiv preprint doi: ; this version posted October 15, 2020. The copyright holder for this preprint (which was not cho has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission.
7
146/1372) and Interferon RR=1.16 (0.96-1.39, p=0.11; 243/2050 vs
216/2050). Unstratified comparisons yielded similarly null findings
(Figure 2), as did analyses excluding corticosteroid users and
multivariate sensitivity analyses estimating simultaneously the effects
of all 4 study drugs (Table S3).
All active treatment ended within ≤14 days, and the numbers of deaths during this 14-day period with any cardiac cause mentioned on the electronic death record was Remdesivir 7v8, Hydroxychloroquine 4v2, Lopinavir 6v3, and Interferon 6v8 (Figure S11). Although many COVID deaths involved multi-organ failure, no study drug death was attributed to renal or hepatic disease.
medRxiv preprint doi: ; this version posted October 15, 2020. The copyright holder for this preprint (which was not cho has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission.
Interpretation should chiefly reflect not the p-value (p=0.21) or point estimate (RR=0.91) but the confidence interval (0.79-1.05), which shows the range of death rate ratios comfortably compatible with the weighted average of the findings from all trials. This absolutely excludes the suggestion that Remdesivir can prevent a substantial fraction of all deaths. The confidence interval is comfortably compatible with prevention of a small fraction of all deaths, but is also comfortably compatible with prevention of no deaths (which would be consistent with the apparent lack of any reduction by Remdesivir in the initiation of ventilation or the duration of hospitalization in Solidarity).
The statistical uncertainties are much greater if attention is restricted to particular subgroups or time periods.9 If Remdesivir has no effect on mortality then chance could still produce somewhat favourable findings in a subgroup of the results for all trials, with more striking findings in a selected subgroup of a particular trial (as in the one subgroup of ACTT-1 where the death rate ratio appeared to be 0.30: Figure 4). Although both ACTT-1 and Solidarity envisaged the possibility of different degrees of benefit in lower- and higher-risk patients, the
For Lopinavir (always co-administered with Ritonavir), the joint mortality RR (combining Solidarity, Recovery and the only informative smaller trial13) was 1.02, 95% CI 0.91-1.14. Although Lopinavir tablets could not be swallowed by ventilated patients, there was no apparent benefit in analyses restricted to those not already being ventilated at entry. This CI indicates no material effect on mortality, and excludes a 10% proportional reduction.
An add-on study within Solidarity, Discovery, recorded many clinical parameters, identifying an unexpected increase in creatinine (perhaps because blood levels are higher than in similarly-dosed HIV patients14,15), but Solidarity and Recovery recorded no renal or hepatic deaths with Lopinavir.
10
interferon was subcutaneous, and subcutaneous and intravenous interferon have different pharmacokinetics,17
For each of these 4 repurposed non-specific antivirals, several thousand patients have now been randomized in
various trials. The unpromising overall findings from the regimens tested suffice to refute early hopes, based on
Cov-2 monoclonal antibodies.
The chief acknowledgement is to the thousands of patients and their families who participated in this trial, and the
and Bin Cao shared Wuhan trial6 details.
MS preparation, revision and submission was controlled by the WHO trial team and the writing committee. There were no
system, blind to study findings. Anonymized data handling and analysis was by the Universities of Berne, Bristol and
Oxford. Remdesivir was donated by Gilead Sciences, Hydroxychloroquine by Mylan, Lopinavir by Abbvie, Cipla and
2.World Health Organization, R&D Blueprint. Informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection
(accessed October 3, 2020).3.World Health Organization, R&D Blueprint. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care
(accessed October 3, 2020).8.Early Breast Cancer Trialists� Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–1717.
medRxiv preprint doi: ; this version posted October 15, 2020. The copyright holder for this preprint (which was not cho has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission.
13.Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid- 19. N Engl J Med 2020;382:1787-99. doi:10.1056/NEJMoa2001282.
14.Venisse N, Peytavin G, Bouchet S et al. Concerns about pharmacokinetic (PK) and pharmacokinetic- pharmacodynamic (PK-PD) studies in the new therapeutic area of COVID-19 infection.
Table 1. Entry characteristics by random allocation, and compliance with that allocation Excludes 64 without cle of each study drug vs concurrent allocation to the same treatment without it. As the control6) is less than the sum of the numbers in the pairwise comparisons. All rights reserved. No reuse allowed without permission.
|
3995 35 | 237 6.2 | 961 | 952 | 335 |
|
511 | 720 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5125 45 | 618 12.8 | 1282 | 410 |
|
597 |
|
934 | |||
| 2146 19 | 398 20.4 | 500 | 469 | 202 | 291 | 396 |
|
Respiratory support
| 1266 11 | 49 3.7 | 287 | 259 | 154 | 235 | 162 |
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
|
8832 78 | 1043 12.7 | 2175 | 656 | 985 | 1723 | ||||
|
1168 10 |
|
281 | 296 | 137 | 179 | 165 |
Geographic location
| 2488 22 | 188 7.8 | 715 | 698 | 286 | 349 | 254 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
1941 17 | 400 22.7 | 470 | 514 | 97 | 96 | 145 |
|
474 | |
| 6837 61 | 665 10.3 | 1558 | 564 | 905 |
|
1322 |
|
| - Chronic liver disease |
|
|---|
Compliance with allocated treatment
% who were taking the study drug midway
% of those reported as discharged
|
69 | 64 | 68 | 55 | ||||
|---|---|---|---|---|---|---|---|---|
| Day 14 | 22 |
|
23 |
|
31 | 19 |
| Day 21 |
|---|
Notes: The few with a particular characteristic unknown are merged with the largest category of that characteristic. �28-d KM %� is the Kaplan-Meier 28-day % risk of in-hospital death. �N�. died� i�c��de� any in-hospital deaths after day 28.
* Interferon randomisation was interferon + Lopinavir vs Lopinavir until 4 July, then it was interferon vs standard of care. ** Albania, Austria, Belgium, Finland, France, Ireland, Italy, Lithuania, Luxembourg, Macedonia, Norway, Spain, Switzerland. § Argentina, Brazil, Colombia, Honduras, Peru.














































































































medRxiv preprint Figursivir, (b) Hydroxychloroquine, doi: ; this version posted October 15, 2020. The copyright holder for this preprint
| 100 | 100 |
|
||
|---|---|---|---|---|
| 15 |
|
|||
| 80 | 10 |  |
Hydroxychloroquine | |
Lopinavir
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate ratio, 1.16 (95% CI, 0.96-1.39) | ||||||||||||||||||||||||||
| 0 | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Lopinavir | 0 | 7 | 14 | 21 | 28 |
|
0 | 7 | ||||||||||||||||||
|
||||||||||||||||||||||||||
 |
7 | 14 | 21 | 28 | ||||||||||||||||||||||
 |
7 | 21 | 28 | |||||||||||||||||||||||
 |
 |
|||||||||||||||||||||||||
| 1399 | 57 | 1333 |
|
1282 |  |
1257 | 1243 | 2050 | 101 | 1669 |  |
1554 |  |
 |
1410 |  |
||||||||||
| Control | ||||||||||||||||||||||||||
| 1372 | 62 | 1293 | 1239 | 1216 | 1203 | Control | 2050 | 91 | 1725 | 1636 | 1498 | |||||||||||||||

























































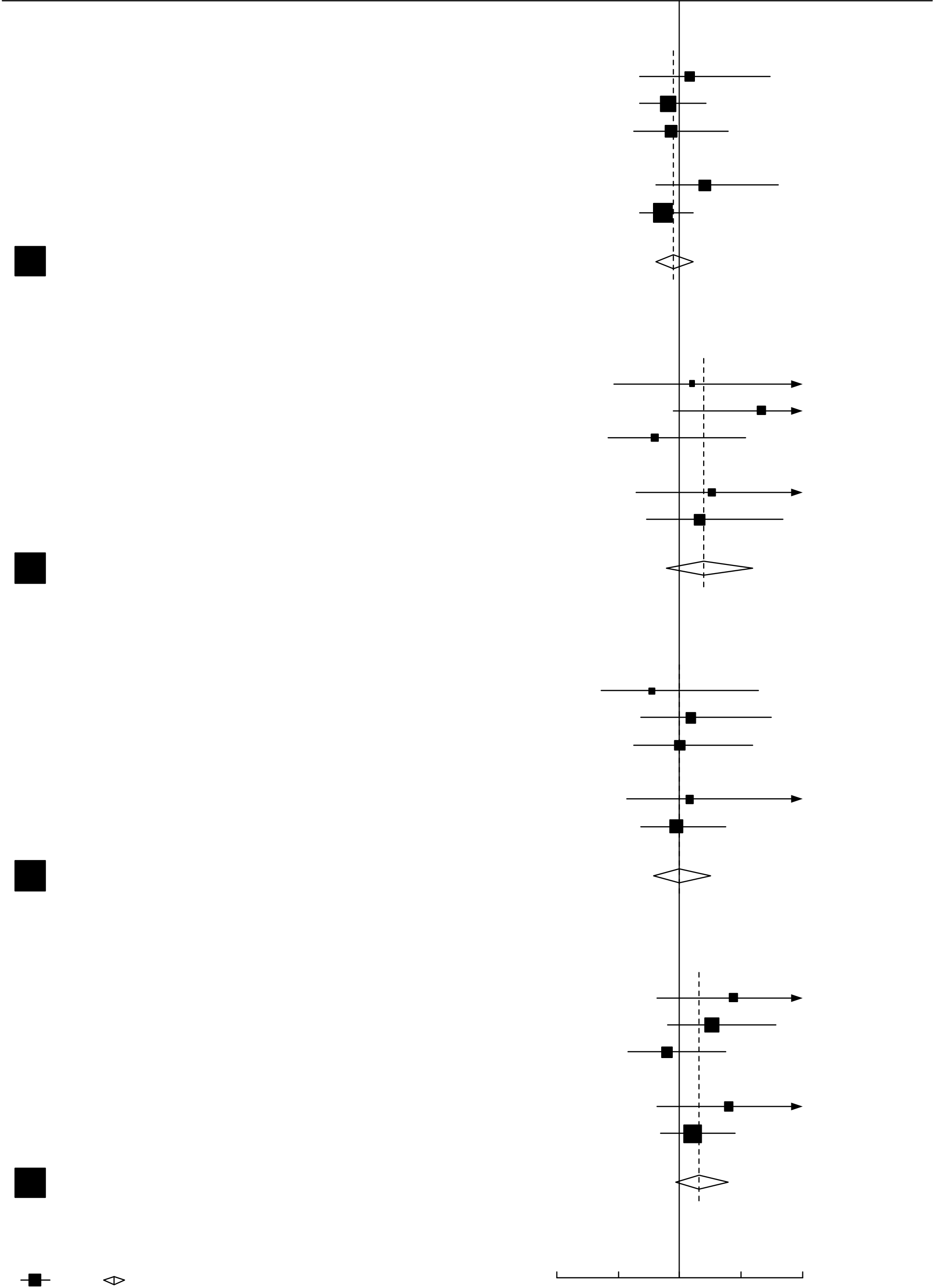







Figure 3. Rate rby age and respiratory support at entry,
| 61/961 (6.9) | 59/952 (6.8) | 2.3 |
|
1.08 [0.67-1.73] | |
|---|---|---|---|---|---|
|
154/1282 (13.8) | 161/1287 (14.2) | -7.6 | ||
| 0.91 [0.68-1.21] | |||||
| 86/500 (20.5) | 83/469 (21.6) | -2.9 | |||
| 0.93 [0.63-1.39] |
(b) Hydroxychloroquine
Age at entry
| 19/335 (5.7) | 19/317 (5.8) | 0.9 | 1.10 [0.47-2.57] | ||
|---|---|---|---|---|---|
| 55/410 (12.1) | 31/396 (7.1) | 10.8 | 1.66 [0.95-2.91] | ||
|
30/202 (14.0) | 34/193 (17.8) | -3.5 |
|
|
| 0.80 [0.42-1.53] |
| 20/511 (3.6) | 27/501 (4.9) | -3.0 | 0.77 [0.36-1.64] | ||
|---|---|---|---|---|---|
|
66/597 (9.8) | 57/596 (9.1) | 2.7 |
|
1.09 [0.68-1.74] |
| 62/291 (20.4) | 62/275 (22.7) | 0.0 | |||
| 1.00 [0.63-1.60] |
|
48/720 (7.5) | 35/697 (5.3) | 7.5 |
|
1.44 [0.82-2.54] |
|---|---|---|---|---|---|
| 122/934 (14.3) | 108/973 (11.4) | 13.3 | |||
| 1.26 [0.90-1.78] | |||||
| 73/396 (19.9) | 73/380 (20.9) | -4.0 | |||
| 0.89 [0.58-1.38] |
Respiratory support at entry
Figure 4. Remdysis of mortality in trials of random allocation
medRxiv preprint doi: ; this version posted October 15, 2020. The copyright holder for this preprint
| Deaths reported / Patients randomized | Ratio of death rates (RR), & | |||
|---|---|---|---|---|
| in ITT analyses (28-day risk, K-M%) | 99% CI (or 95% CI, for total) | |||
|
Control |
|
Remdesivir | |
Subtotals
|
231/3309 (7.0) | 282/3277 (8.6) | -27.6 | 0.80 [0.63-1.01] | |
|---|---|---|---|---|---|
| 156/509 (30.6) | 126/505 (25.0) | 10.1 | 1.16 [0.85-1.60] | ||
| Total |
|
|
0.91 [0.79-1.05] | ||
�he HR�� CI). RR i� g�� b� �a�i�g ��geRR to be (O-E)/V with Normal variance 1/V. Subtotals


