Middle rostral and caudal oen neurons isl gfp fish and dpf
4Maxim Nikitchenko, 5,6James A. Gagnon, 7Pablo Oteiza, 1,2Richard Schalek, 1Adi Peleg,
8,9,10Ruben Portugues, 1,2,#Jeff Lichtman, 1,2,#,+Florian Engert
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
Abstract
state-dependent modulation found at later stages in development.
2
| motor-command | centers |
|---|
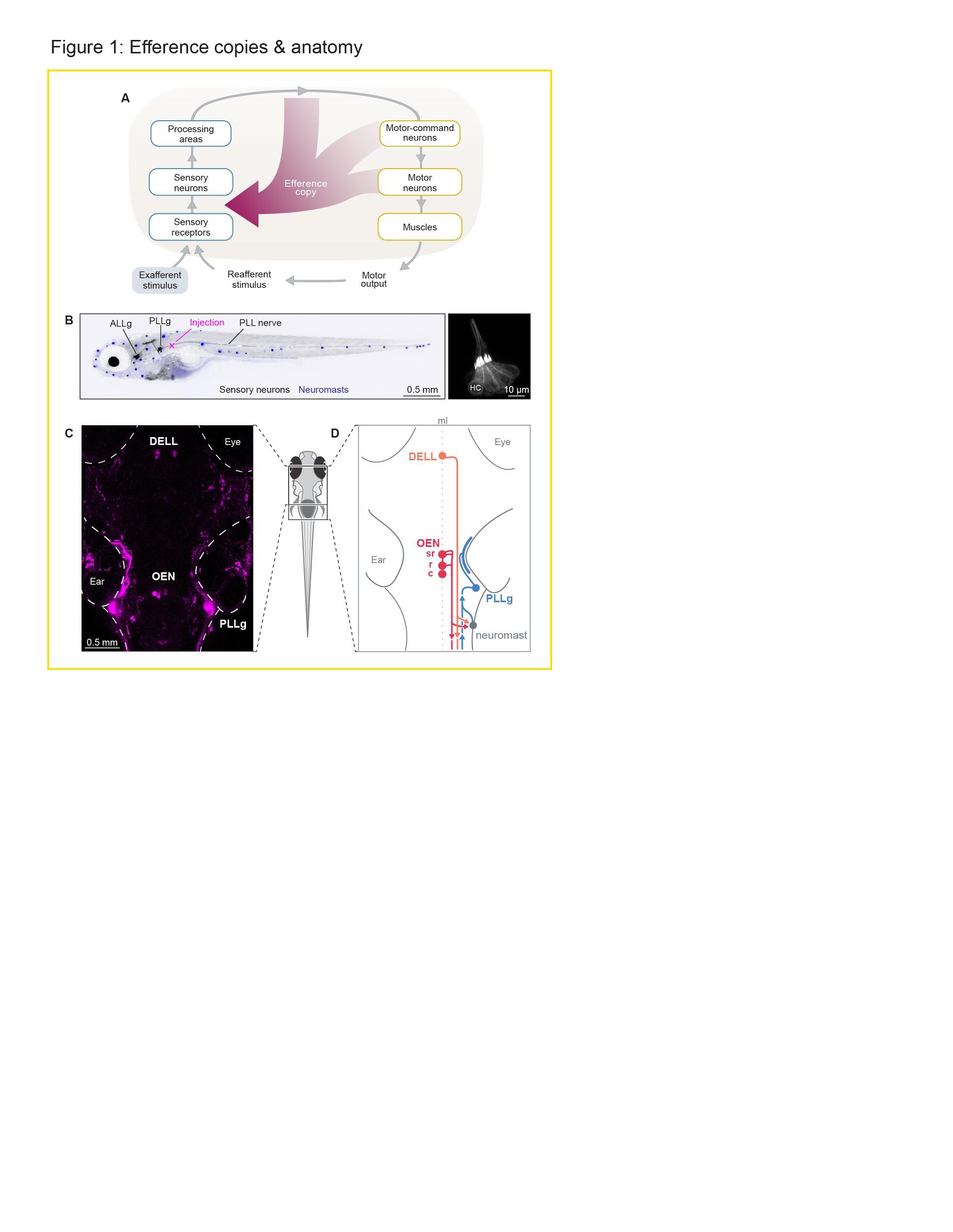
One of the earliest detailed dissections of the neural mechanisms underlying reafferent cancellation was accomplished in weakly electric fish, so named for their ability to emit electrical discharges. Electrosensory receptor organs located in the lateral line detect the resulting electric field lines, and distortions in these fields are used for electrolocation by dedicated downstream circuits in the brain. Notably, the self-generated electrical pulses themselves are adaptively cancelled out by cerebellar-like structures precisely tuned to subtract a copy of the expected incoming sensory information in a flexible and dynamic fashion .
Most fish and amphibia lack such specialized electrosensory organs and use the lateral line exclusively to sense water motion relative to their bodies . This information can be used to identify moving animals in their vicinity, as well as abiotic water currents, and therefore contributes to a variety of behaviors including schooling , prey capture , predator avoidance , and rheotaxis . Because fluid drag during locomotion strongly activates the lateral line, in this case too, external and self-generated stimuli need to be processed differentially.
hindbrain and the other from the Dopaminergic Efferent to the Lateral Line (DELL) in the ventral hypothalamus . Further, acetylcholine has been shown to inhibit hair cell activity , making the OEN the most likely source of reafferent suppression. At the synaptic level, ideas about how acetylcholine may inhibit hair cells come from work done in the mammalian inner ear. In the rat cochlea, the hyperpolarizing effects of acetylcholine result from the activation of nicotinic receptors containing specialized α9/α10 subunits . This inhibitory effect, however, is less related to reafferent suppression and is thought to have a protective function when animals are exposed to acoustic hyperstimulation that can induce damage due to excitotoxicity . Interestingly, enriched expression of the α9, but not the α10 subunits, have been observed in the hair cells of the lateral line of larval zebrafish , but whether they play a specific role in protective silencing, context-dependent modulation or treafferent suppression has not yet been determined. Nonetheless, there is now significant evidence for the role of cholinergic efferents in reafferent suppression, and their mechanism has been hypothesized to the level of individual receptor subtypes. The functional role and mechanism of the dopaminergic efferent neurons, on the other hand, is still very much unclear.
It is further unknown how the convergence of all of the involved cell populations, namely the two efferent subtypes (OEN and DELL), the afferent sensory neurons, and the hair cells of the neuromasts, gives rise to an interconnected and complex microcircuit that can adaptively process the sensory signals detected by the lateral line.
4
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
5
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
Overall, tracing of individual efferent neurons revealed a large degree of divergence that is not somatotopically organized. Since neuromasts in different parts of the body can receive inputs from the same efferent neurons and will likely experience temporally synchronized stimuli during swimming, it is likely that the efferent mechanisms in place act in bulk, rather than being finely tuned to different regions of the body.
In summary, we confirmed the existence of two sources of descending inputs to the hair cells of the lateral line of zebrafish larvae. The first, the DELL, is a dopaminergic hypothalamic nucleus and the second, the OEN, is a cholinergic nucleus with further anatomical subdivisions. Both nuclei are anatomically well-poised to provide sensory modulation, and could transmit efference copy signals to compensate for self-generated stimulation during locomotion.
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
Functional properties of afferent and efferent neurons during locomotion
| directionally | tuned | to | two | flow | directions: | head-to-tail | or | tail-to-head |
|---|
To complement this observation, we measured the responses of lateral line sensory neurons during swimming in the absence of exafferent stimulation. Since zebrafish larvae have a low motor drive in our head-embedded preparation, we used a black and white moving grating to elicit the optomotor response (OMR) . In this way, we focused our analysis on the sensory activity during visually-evoked swim events where the flow patterns detected by the lateral line are exclusively generated by the fish’s own motion (reafferent stimulation) (Figure 2E). Remarkably, even though hair cells are strongly deflected by fluid drag during tail undulations , we observed that self-induced flow stimuli did not result in sensory neuron activation during locomotion (Figures 2F and 2G).
These experiments show that whilst sensory neurons in the PLLg can be activated by exafferent mechanosensory stimuli, they are not excited by hair cell deflections during swims, favoring the hypothesis that efference copy signals exist in the lateral line system of larval zebrafish, and that these are transmitted directly to the peripheral sensory pathway.
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
DELL and OEN neurons exhibit graded motor-correlated activity, while DELL neurons are also activated by sensory stimuli.
We next focused our attention on the OEN and observed that these cholinergic neurons also exhibit elevated activity during swims and startle responses (Figures 3G-H, 3J and S2H-I). However, in stark contrast to the DELL, OEN neurons responded exclusively during motor events and were not activated by any sensory stimulus in the absence of locomotion (Figures 3I-L and S2I). In accordance with this observation, ablation of the lateral line by copper treatment did not affect OEN responses in any way (Figure S2J), and OEN activity was indistinguishable during spontaneous and visually-evoked swims (Figure S2K). This pronounced coincidence with—and selectivity for—motor activity provides support for a primary role of this nucleus in efference copy transmission.
11
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
Since different behaviors generate different patterns of reafferent stimulation, and would thus require different patterns of efferent cancellation, we tested whether the activity of the OEN subnuclei differs when the animal executes distinct motor programs. To that end, we compared activity evoked by startles and swim bouts across the three OEN subnuclei. These two behaviors differ in that short-latency (<40 ms) startle responses are generated by Mauthner array cells , while routine swims are controlled by other reticulospinal neurons . We found that in spite of this distinct segregation of motor control units, OEN neurons in all subnuclei are synchronously active both during swims and startle responses (Figures S2L and S2M). This suggests that all three OEN subgroups can be thought of as one functional unit for the behaviors tested.
| and | startle | responses. | DELL | neurons, | however, | are | also | activated | by | visual |
|---|
13
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which
Figure S2. Activity of efferent nuclei during locomotion and in response to diverse sensory stimuli. Related to Figure 3.
(A-D) Top: Population averages of DELL neuronal activity (mean ΔF/F ± s.e.m.). Bottom: swim probability. (A) Average swim-triggered neuronal responses (n=6 fish expressing GCaMP6f).
(F) Mean percentage of DELL cells per fish whose activity was significantly correlated with maximum tail amplitude or tail beat frequency (n=9 fish, dark gray p < 0.001, light gray < 0.05, error bars: s.e.m).
(G) Scatter plots showing the relationship between the power of each individual swim bout versus the intensity of the concurrent neuronal responses of all labeled DELL neurons in each of the 9 fish analyzed. Correlation coefficients and best-fit lines arising from linear regression were calculated for each neuron, and used to calculate the mean correlation coefficient (r) per fish. Swim power was defined as the integral of the absolute tail curvature trace for individual bouts, and neuronal activity as the integral of the calcium transient during each corresponding bout. (H-K) Top: Population averages of OEN neuronal activity (mean ΔF/F ± s.e.m.). Bottom: swim probability.
(O) Mean percentage of OEN cells per fish whose activity was significantly correlated with maximum tail amplitude or tail beat frequency (n=10 fish, dark gray p < 0.001, light gray < 0.05, error bars: s.e.m).
(P) Scatter plots showing the relationship between the power of all individual swim bouts versus the neuronal responses of all labeled OEN neurons in each of the 10 fish analyzed. Correlation coefficients and best-fit lines arising from linear regression were calculated for each neuron (shown in different shades), and used to calculate the mean correlation coefficient (r) per fish. Swim power was defined as the integral of the absolute tail curvature trace for individual bouts: a stationary tail has little curvature and thus power is ~0, whereas an undulating tail has a positive absolute tail curvature, which increases as a function of motor strength. Neuronal activity was defined as the integral of the calcium transient during each corresponding bout.
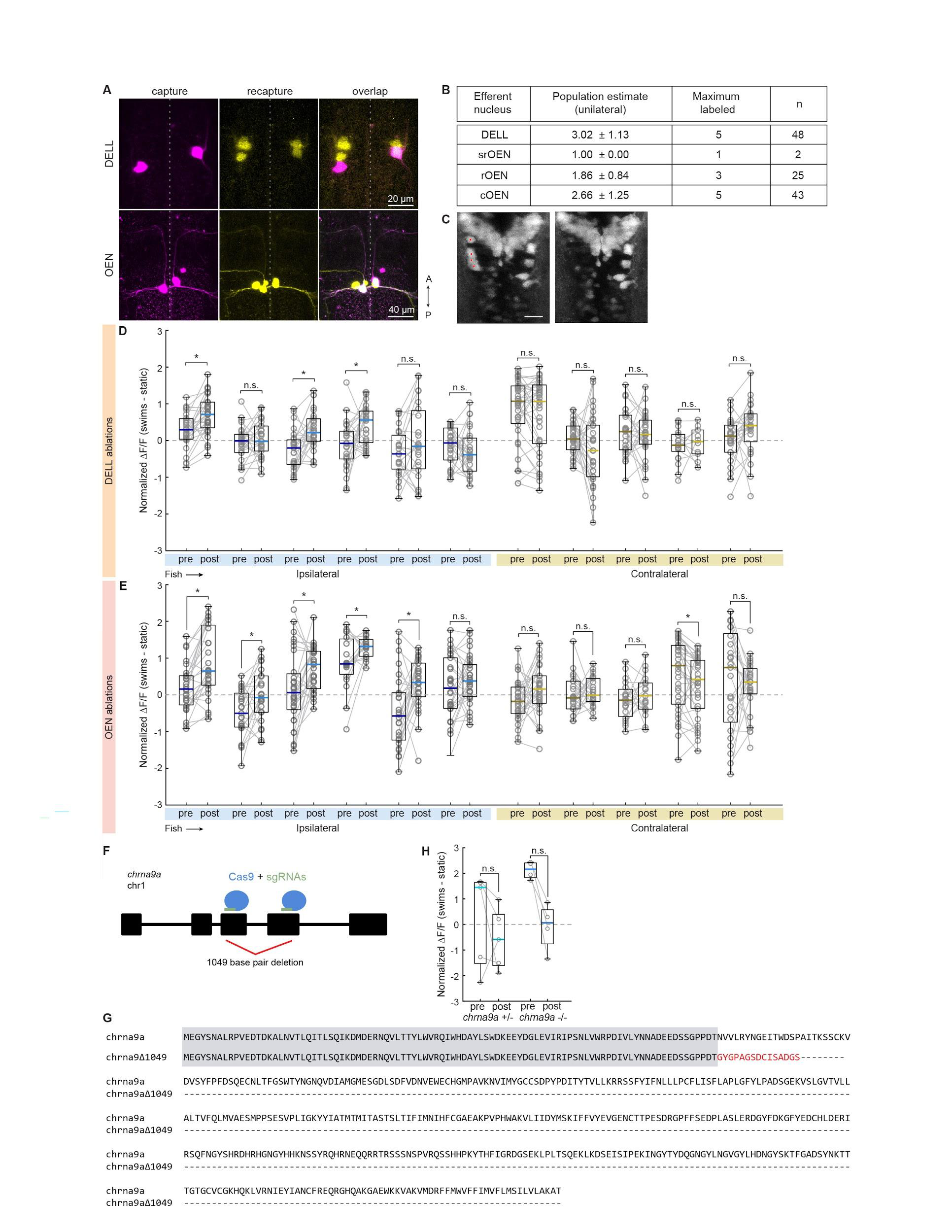
In a single neuromast, we found 7 pairs of hair cells of opposing polarity (half rostro-caudal and half caudo-rostral). Since sterocilia in the hair cell bundle are organized in increasing length towards the kinocilium, polarity was determined by noting this asymmetry. We also detected multiple neurites: 6 afferent neurites selectively targeting one of the hair cell polarities, 3 axonal arbors originating from three separate DELL neurons and 2 axonal arbors originating from distinct OEN neurons (Figure 4A; Movie S3). Additionally, two neurites lacked counterparts in the confocal microscopy data, so their identities or origin could not be assigned (Movie S3). The complete matrix quantifying the pairwise connectivity of all circuit elements is shown in Figure 4G.
The simplest hypothesis about the “micro-connectome” within this region is that hair cells form excitatory connections onto the afferent terminals of sensory neurons, whereas DELL and OEN terminals contact the hair cells with dopaminergic and cholinergic synapses respectively. Previous ultrastructural studies in zebrafish uncovered that hair cells connect with afferent sensory neurons via ribbon synapses , and that afferent neurons selectively innervate hair cells of a single polarity . Our ultrastructural volumetric analysis corroborated this specificity: three of the afferent neurites received inputs from rostro-caudally-tuned hair cells exclusively, and the other three were targeted by hair cells of the opposite polarity (Figures 4A and 4G).
(A) Innervation of a posterior lateral line neuromast. Neuron identities were assigned by correlating anatomical ssEM data to fluorescent labeling of efferent types in the same neuromast. This neuromast consists of 14 hair cells belonging to two equal populations of opposing hair-bundle polarities (rostro-caudal and caudo-rostral). (B-E) EM details showing examples of specific connections. (B) Hair cells (HC) and afferent neurons (AF) connect via ribbon synapses (right arrowhead). An efferent (EF) OEN terminal containing synaptic vesicles abuts the base of the hair cell, which contains postsynaptic membrane specializations (left arrowhead). (C) A vesicle-rich dopaminergic efferent terminal in the proximity of a hair cell. Note the distance between the efferent membrane and the hair cell, as well as the presence of intercalated support cells (SC). (D) A DELL efferent axon with vesicle-filled profile within the axon bundle innervating the neuromast. The vesicle-filled profile is in close apposition to afferent neurites carrying information from both polarities. (E) An OEN efferent axon with vesicle release sites, in close apposition to an afferent neurite within the axon bundle that innervates the neuromast. (F) Histogram showing the distances between DELL (orange) or OEN (red) vesicle sites and the closest hair cell. (G) Connectivity matrix summarizing the tally of all synaptic contacts observed in this neuromast (see methods for quantification).
17
(A) DELL vesicle-filled profiles in close apposition to an OEN axon (arrowheads), surrounded by a support cell (SC). (B) Vesicle-filled profiles from two separate DELL neurons contact each other. A hair cell (HC) is also labeled for reference. Scale bars: 1 μm.
18
Next, we tested the effect of silencing the OEN efferent pathway on PLLg sensitivity. Here, we found that before ablations, sensory neurons in the labeled and control ganglia were silent both during periods of quiescence and during motor events, as expected (Figures 5D-F). Strikingly, after OEN ablations, a subset of neurons in the ipsilateral PLLg displayed robust responses during swimming, demonstrating that OEN input is necessary for sensory inhibition during locomotion (Figures 5E, 5F and S4E, blue). All neurons of the contralateral ganglion, on the other hand, continued to be silent during swim bouts (Figures 5E, 5F and S4E, yellow). The fact that a subset of ipsilateral PLLg neurons remained silent during swims indicates that some inhibition was preserved. This can be readily explained by the partial and stochastic nature of our ablation method, which on average left about three OEN neurons unlabeled and consequently intact in each animal. These functional results are fully in line with the ultrastructurally-determined connectivity patterns we have observed in Figure 4: individual hair cells do not receive inputs from all OEN neurons, but rather, are targeted stochastically by a small subset. Therefore, a partial removal of OEN neurons is expected to uncover activity only in a subset of PLLg neurons and leave the rest fully inhibited. Furthermore, this could also be explained by the observation that whilst all hair cells in a neuromast are mechanosensitive, a
19
manipulations, that the cholinergic efferent pathway plays a critical role in silencing reafferent activity in the lateral line, and that this inhibitory action is precisely synchronized with the occurrence of locomotor events. The role of the dopaminergic pathway, on the other hand, is less clear. We did not observe any obvious modulatory effects of dopaminergic efferents on sensory neuron activity, but our ultrastructural analysis revealed synaptic contacts between efferent dopaminergic axons and afferent sensory neurites. It is possible that the modulatory effect of dopamine occurs over longer time-spans that precludes analysis in our experimental settings.
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
The Efferent Nuclei
We have identified the OEN and DELL as carriers of corollary discharges, as they both fire in strict synchrony with motor actions such as swims and escapes. However, only the OEN is shown to cancel out the predicted reafferent stimulation through direct inhibition of the hair cells via specialized nicotinic receptors.The exact role of the DELL neurons remains unclear, but their dopaminergic nature together with their targeting of afferent neurites is suggestive of a role in sensory processing modulation. Furthermore, previous work has shown that dopamine can have a sensitizing effect on neuromast hair cells via the D1b receptor . The authors, however, did not detect D1b receptor expression in any of the neurons that innervate hair cells, so the molecular players in the synapses between DELL axons and afferent neurites are still unknown. Since DELL neurons exhibit increased activity during locomotion, and also in response to sensory stimuli such as moving visual gratings, flow and waves elicited by taps (Figures 3A-F), their effects are dependent on both motor-state and salient sensory stimulation . By virtue of its action through G-protein coupled receptors, the effects of dopamine likely outlast the brief occurrences of swims or stimuli—leaving the sensory system in a more receptive state during the inter-bout periods or following stimuli that might require subsequent behavioral responses. Additionally, DELL neurons send collaterals to the spinal cord, and have been shown to affect bout frequency, indicating a role in the regulation of spinal circuit excitability . Taken together, these observations suggest that DELL neurons serve a dual function in the control of basal threshold levels of both sensory and motor networks.
The Connectome of the Neuromast
Seminal work on the lateral line of zebrafish larvae provided the first EM images of a neuromast and established the existence of synaptic connections between hair cells and sensory afferents, and efferents and hair cells .
| Subsequently, | a | comprehensive |
|
of | larval | zebrafish | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| performed | at | an | early | developmental | stage | (~3 | ||||||
Since cholinergic terminals form direct synapses onto hair cells, the average distance between their respective membranes was, as expected, less than 100 nm (Figure 4F). For the majority of dopaminergic release sites, on the other hand, this distance exceeded 500 nm. This suggested at first glance, and in line with previous light microscopy work , that
24
A Complete Model of Local Microcircuitry
We show here that larval zebrafish possess parallel descending inputs that can differentially influence mechanosensory processing at early stages within the sensory pathway. Cholinergic signals from the hindbrain transmit efference copy signals that cancel out
I.O. and F.E. conceived of the project and designed the experiments with contributions from R.P., M.H. and P.O. I.O. performed the experiments, using software written by R.P. in the behavioral set-up, and in collaboration with M.H. for experiments with heat delivery, and M.N. with flow. M.D.P. obtained and analyzed the neuromast connectome with support from J.B-W. for tissue preparation and tracing, J.B-W. and R.S. for data acquisition, A.P. for image alignment and volume reconstruction, and J.L. for infrastructure and general supervision. J.A.G. generated the chrna9a mutants. I.O. analyzed the data, and wrote the paper with F.E. All authors read and commented on the manuscript.
Acknowledgments
| , | and | Tg(elavl3:H2B-mCherry) | to | label | neuronal | nuclei. | For | functional |
|
|---|
experiments, larvae homozygous for the Tg(elavl3:GCaMP6s) or Tg(elavl3:GCaMP6f) were used. In most cases, the animals were also homozygous for the mitfaw2/w2skin-pigmentation . Fish were raised in fish facility water on a 14/ 10 hr light/dark cycle at 28 °C. Larvae were fed Paramecia daily from 5 dpf. Animal handling and experimental procedures were approved by the Harvard University Standing Committee on the Use of Animals in Research and Training.
Capture-recapture random sampling.
Neurons were tagged using the dye labelling technique described above. The ‘capture’ step involved injecting Cascade Blue-conjugated dextrans (3,000 MW, Invitrogen) at the level of the L1 neuromast, at 5 dpf. 48 hs were allowed for nerve regeneration . In the
| 𝑁 =(𝑚2+1)(𝑚1+1) 𝑜+1 |
|---|
nuclear-targeted mCherry to aid in counting neurons, and Tg(Isl1:GFP) to have anatomical landmarks to distinguish between the three hindbrain nuclei.
27
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
| micropipettes | were | placed | near | the | efferent | nuclei, | which | were | visualized |
|---|
In vivo 2-photon functional imaging.
A custom built 2-photon microscope was used for all functional experiments. The laser, a Ti:Sapphire ultra-fast laser (MaiTai, Spectra-Physics) was tuned to 950 nm, and operated at an average laser power of 5-10 mW at sample. Images were collected by scanning frames at 4 Hz and consecutive planes were separated by 2 μm. Image acquisition was controlled using custom software written in LabView (National Instruments).
28
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
Data analysis.
Data analysis was performed using scripts written in MATLAB (MathWorks).
20th percentile of its entire fluorescence signal per plane. Perceptually uniform color maps developed by Ander Biguri were used to graph the average single-cell responses in Figure 2. https://www.mathworks.com/matlabcentral/fileexchange/51986-perceptually-uniform-colormaps To compute swim-triggered averages and for analyses correlating neuronal activity to different features of swim kinematics (Figure 3M-N, S2E-G and S2N-P), only ‘unitary’ bouts were taken into account. That is, bouts that occurred after at least 4 s from a previous bout and that were not followed by a subsequent bout within 2.5 s. The selected swim features were (1) Swim power, defined as the integral of the absolute tail curvature trace for an individual bouts, as in . (2) Maximum amplitude, the maximum tail curvature achieved per bout and (3) Tail beat frequency, the inverse of the time between successive extreme tail positions in the same direction, as in . Neuronal activity was defined as the integral of the
29
Fish were incubated in 1 mM copper sulfate for 85 min and allowed to rest in fish facility water for 60 min. Only animals that showed complete neuromast ablation (assessed by DiASP staining: 0.5 mM in fish facility water for 15 min, as in were used for additional functional imaging assays.
Tissue preparation for ssSEM.
| imaged | under | a | confocal | microscope | to | capture | the | pattern | of | innervation | of |
|---|
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
overnight), embedded at 60 °C for 72hrs. The cured block was trimmed, mounted, cut in 30 nm thick sections and imaged as in .
Fish were subjected to unilateral dye injections in the lateral line at 4 dpf as described above, and allowed to recover in freely-swimming conditions for 48 hs to allow for nerve regeneration after injury . At 6 dpf, fish were embedded in low-point melting agarose, and the agarose surrounding their tails was removed. Functional experiments were performed the following day. After a round of baseline functional experiments, we performed the ablation procedure as described in , with the exception that anesthesia was not used. Individual efferent neurons were targeted systematically by receiving 1-3 850 nm laser pulses of 1 ms, at 80 % laser power. Fish were then immediately used for functional experiments to test for the effects of ablations on sensory processing. On average, 4 OEN efferent neurons were ablated from the 8 that are hypothesized to exist (Figure S4B).
Generation of chrna9a mutants using CRISPR–Cas9.
chrna9a_target4 CGTACACAGTCCTGCTCAAGCGG
chrna9a_target5 GTGGAGCCAAGAAAGAGATGAGG
chrna9a_flanking_PCR_R ACCATCAGCTGAAATACAGTCAGAG
Statistics
correlation was computed. When comparing fish populations of multiple genotypes (Figure 5I),
the nonparametric Kruskal-Wallis one-way analysis of variance (ANOVA) tes was used, followed
Movie S2. Neuronal-type identification via correlation of confocal and EM volumes. Related to Figure 4. Efferent neuronal identities were assigned by correlating EM images to confocal fluorescent images obtained prior to fixation. In addition to the assigned afferent and efferent neurons, there are two neurons with unassigned identities: (i) putative afferent neuron (blue) which does not form contacts of any kind, has no synaptic vesicles and becomes myelinated within the nerve bundle, and (ii) putative dopaminergic efferent neuron, which resides near the base of the hair cells, does not form contacts with the hair cells and has synaptic vesicles, but is not observed in the confocal image stack.
Movie S3. Volumetric recontrunstruction of a neuromast. Related to Figure 4. Full segmentation of the lateral line neuromast. All hair cells and innervating neurons have been densely segmented. Additionally, some support cells have been segmented (green shade) and the layer of skin collagen (blue) is shown.
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
34
bioRxiv preprint doi: ; this version posted May 6, 2021. The copyright holder for this preprint (which was nnder. All rights reserved. No reuse allowed without permission.
37
Berger, D.R., Sebastian Seung, H., and Lichtman, J.W. (2018). VAST (Volume Annotation and Segmentation Tool): Efficient Manual and Semi-Automatic Labeling of Large 3D Image Stacks. Frontiers in Neural Circuits 12.
Bodznick, D., Montgomery, J.C., and Carey, M. (1999). Adaptive mechanisms in the elasmobranch hindbrain. J.Exp.Biol. 202, 1357–1364.
Cahn, P.H., and Shaw, E. (1965). A Method for Studying Lateral Line Cupular Bending in Juvenile Fishes. Bull. Mar. Sci. 15, 1060–1071.
Carpaneto Freixas, A.E., Moglie, M.J., Castagnola, T., Salatino, L., Domene, S., Marcovich, I., Gallino, S., Wedemeyer, C., Goutman, J.D., Plazas, P.V., et al. (2021). Unraveling the Molecular Players at the Cholinergic Efferent Synapse of the Zebrafish Lateral Line. J. Neurosci. 41, 47–60.
Cullen, K.E. (2004). Sensory signals during active versus passive movement. Curr. Opin. Neurobiol. 14, 698–706.
Dawkins, R., Keller, S.L., and Sewell, W.F. (2005). Pharmacology of acetylcholine-mediated cell signaling in the lateral line organ following efferent stimulation. J. Neurophysiol. 93, 2541–2551. Dijkgraaf, S. (1963). The functioning and significance of the lateral-line organs. Biol. Rev. Camb. Philos. Soc. 38, 51–105.
Erickson, T., and Nicolson, T. (2015). Identification of sensory hair-cell transcripts by thiouracil-tagging in zebrafish. BMC Genomics 16, 842.
Faucherre, A., Pujol-Martí, J., Kawakami, K., and López-Schier, H. (2009). Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS One 4, e4477.
Ghysen, A., and Dambly-Chaudière, C. (2007). The lateral line microcosmos. Genes Dev. 21, 2118–2130.
Haehnel-Taguchi, M., Fernandes, A.M., Böhler, M., Schmitt, I., Driever, W., and Others (2018). Projections of the Diencephalospinal Dopaminergic System to Peripheral Sense Organs in Larval Zebrafish (Danio rerio). Front. Neuroanat. 12, 20.
von Holst, E., and Mittelstaedt, H. (1950). Das Reafferenzprinzip. Naturwissenschaften 37, 464–476.
Inoue, D., and Wittbrodt, J. (2011). One for All—A Highly Efficient and Versatile Method for Fluorescent Immunostaining in Fish Embryos. PLoS One 6, e19713.
Labun, K., Montague, T.G., Gagnon, J.A., Thyme, S.B., and Valen, E. (2016). CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44, W272–W276.
Lacoste, A.M.B., Schoppik, D., Robson, D.N., Haesemeyer, M., Portugues, R., Li, J.M., Randlett, O., Wee, C.L., Engert, F., and Schier, A.F. (2015). A convergent and essential interneuron pathway for Mauthner-cell-mediated escapes. Curr. Biol. 25, 1526–1534.
Liu, K.S., and Fetcho, J.R. (1999). Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23, 325–335.
López-Schier, H., Starr, C.J., Kappler, J.A., Kollmar, R., and Hudspeth, A.J. (2004). Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev. Cell 7, 401–412.
Montgomery, J.C., and Bodznick, D. (1994). An adaptive filter that cancels self-induced noise in the electrosensory and lateral line mechanosensory systems of fish. Neurosci. Lett. 174, 145–148.
Montgomery, J.C., Macdonald, F., Baker, C.F., and Carton, A.G. (2002). Hydrodynamic contributions to multimodal guidance of prey capture behavior in fish. Brain Behav. Evol. 59, 190–198.
Oteiza, P., Odstrcil, I., Lauder, G., Portugues, R., and Engert, F. (2017). A novel mechanism for
41
Pichler, P., and Lagnado, L. (2020). Motor Behavior Selectively Inhibits Hair Cells Activated by Forward Motion in the Lateral Line of Zebrafish. Curr. Biol. 30, 150–157.e3.
Pitcher, T.J., Partridge, B.L., and Wardle, C.S. (1976). A blind fish can school. Science 194, 963–965.
Russell, I.J. (1971). The role of the lateral-line efferent system in Xenopus laevis. J. Exp. Biol. 54, 621–641.
Russell, I.J. (1976). Central inhibition of lateral line input in the medulla of the goldfish by neurones which control active body movements. J. Comp. Physiol. 111, 335–358.
Schuster, K., and Ghysen, A. (2013). Labeling hair cells and afferent neurons in the posterior lateral-line system of zebrafish. Cold Spring Harb. Protoc. 2013, 1172–1174.
Severi, K.E., Portugues, R., Marques, J.C., O’Malley, D.M., Orger, M.B., and Engert, F. (2014). Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83, 692–707.
Stewart, W.J., and McHenry, M.J. (2010). Sensing the strike of a predator fish depends on the specific gravity of a prey fish. J. Exp. Biol. 213, 3769–3777.
Straka, H., Simmers, J., and Chagnaud, B.P. (2018). A New Perspective on Predictive Motor Signaling. Curr. Biol. 28, R232–R243.
Tay, T.L., Ronneberger, O., Ryu, S., Nitschke, R., and Driever, W. (2011). Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat.Commun. 2, 171.
Toro, C., Trapani, J.G., Pacentine, I., Maeda, R., Sheets, L., Mo, W., and Nicolson, T. (2015). Dopamine Modulates the Activity of Sensory Hair Cells. J. Neurosci. 35, 16494–16503.
Wen, L., Wei, W., Gu, W., Huang, P., Ren, X., Zhang, Z., Zhu, Z., Lin, S., and Zhang, B. (2008). Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 314, 84–92.
43
Zipser, B., and Bennett, M.V. (1976). Interaction of electrosensory and electromotor signals in lateral line lobe of a mormyrid fish. Journal of Neurophysiology 39, 713–721.
44