The similar slopes the potential energy curves generated the
| 张 | 马 |
|---|
(洛阳师范学院化学化工学院, 河南 洛阳 471022)
摘要: X―H···Y (Y为电子供体)型氢键形成时, X―H键长伸长或缩短与相应的X―H伸缩振动频率红移或蓝 移存在较强的相关性, 这也是氢键光谱检测和研究的基础. 但是, 最近的理论研究却推翻了这一观点, 认为X―
| ZHANG Yu | MA Ning |
|---|
Abstract: The correlation between the X―H bond-length change and the corresponding X―H stretching frequency shift upon X ― H···Y (Y is an electron donor) hydrogen bond formation is the basis for the spectroscopic detection and investigation of the hydrogen bond. However, this view has been questioned in a recent report, suggesting that the widely accepted correlation between the bond-length change and the frequency shift in hydrogen-bonded complexes is unreliable (McDowell, S. A. C.; Buckingham, A. D. J. Am. Chem. Soc. 2005, 127, 15515.). In this work, several robust computational methods have been used to investigate this issue. The results clearly show that a computational artifact leads to the conclusion incorrectly reported by McDowell and Buckingham and that the correlation between the X―H bond-length change and the corresponding X―H stretching frequency shift is still very good in the hydrogen-bonded complexes studied.
frared and Raman spectroscopies have played important roles in the detection of the hydrogen bonds: conventional X―H···
Received: November 4, 2011; Revised: December 27, 2011; Published on Web: December 30, 2011.
|
|---|
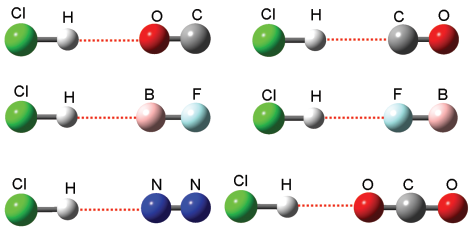
Fig.1 Linear complexes considered in this work The red dot lines represent the hydrogen bonds.
| Fig.2 |
|
|---|
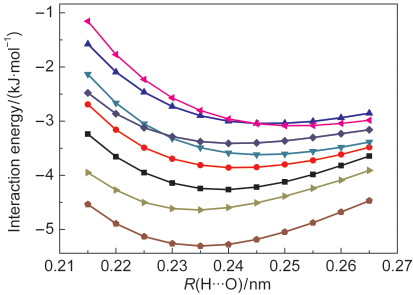
QCISD/6-311++G(2d,2p) and QCISD/6-311++G(3df,3pd) lev-els of theory are inaccurate relative to the CCSD(T)/CBS benchmark, and furthermore they can not produce the correct results of the bond-length change and the frequency shift. Let us add here that McDowell and Buckinghamʹs conclusions are based on the QCISD/6-311 ++ G(2d,2p) and QCISD/6-311 ++ G(3df,3pd) calculations. 5 In comparison with the CCSD(T)/ CBS benchmark, the similar slopes of the potential energy curves generated at the M05-2X/6-311 ++ G(3d,3p), M06-2X/ 6-311++G(3d,3p), B2PLYP-D/6-311++G(3d,3p), and mPW2PL-YP-D/6-311++G(3d,3p) levels of theory confirm the reliability of the density functionals M05-2X, M06-2X, B2PLYP-D, and mPW2PLYP-D for the study of the bond-length change-fre-quency shift correlation of the hydrogen-bonded complexes considered in the present study.
|
3.2 |
|---|
| 0.12744 | 0.12772 | 0.12855 | 0.12736 | 0.12749 | 0.12755 | |
|---|---|---|---|---|---|---|
| 0.23720 | 0.24164 | 0.23663 | 0.23917 | 0.24346 | 0.22376 | |
| 3004.7 | 2956.1 | 2817.8 | 3013.2 | 2997.1 | 2994.9 | |
|
144 | 244 | 549 | 113 | 163 | 197 |
| μ | 4.6942×10-30 | 5.3284×10-30 | 9.3925×10-30 | 1.5674×10-30 | 4.8247×10-30 | 5.4885×10-30 |
| ΔE CP | 3.85 | 6.82 | 13.85 | 1.21 | 4.10 | 6.99 |
6-311++G(3df,3pd)
| 502 | Acta Phys. ⁃Chim. Sin. 2012 | Vol.28 |
|---|
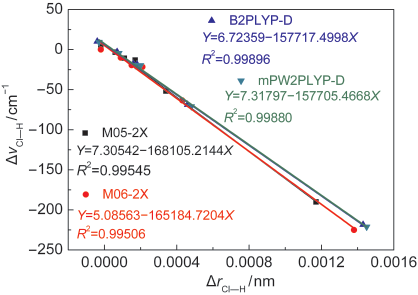
Fig.3 Correlation of Cl―H bond-length change with Cl―H stretching frequency shift in hydrogen-bonded complexes
| 503 | ||
|---|---|---|
| S.; Alkorta, I.; Clary, D. C.; Crabtree, R. H.; Dannenberg, J. J.; | ||