CH7003 Practise Problem 3
1. Consider the chain reaction A -> B + C which proceeds by the steps:
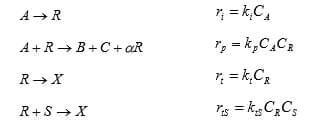
The second termination step occurs by reaction of a scavenger S. Find the expression for the rate of this reaction in terms of CA, CS and ki, kp, kt, ktS. Assuming CR is small. Under what condition does the reaction reach the maximum possible reaction orders with respect to CA?
The reaction H2 + O2 -> H2O proceeds first via adsorption of H2 and O2 as atoms. It has a rate-limiting step H - S + O -S -> HO - S (here S stands for the surface active sites) and the rate of the reaction is r = kRΘHΘo. Derive a reaction rate expression r(PH2 ,PO2 KH2 ,KO2 ) for this reaction assuming negligible coverage of HO and H2O and a Langmuir Hinshelwood kinetics with competitive adsorption, where Ki is the adsorption-desorption equilibrium constant.
3. The irreversible, gas-phase reaction 2A B+C is conducted over a solid catalyst. The reaction may be considered isothermal. The following reaction mechanism is proposed, where S represents a surface site.
(i) A + S A-S
(ii) A-S + A-S B-S + C + S
(iii) B-S B + S
It is proposed that step (ii) is rate-determining and irreversible, whereas steps (i) and (iii) are equilibrated.
- Based on the proposed mechanism, derive the LH rate expression for the reaction.
- Experiments with a differential reactor have been used to measure the reaction rate, tabulated in the table below.
|
PA |
PB |
PC |
Measured Rate |
|
1.5 |
0.0 |
0.0 |
9.9 |
|
1.5 |
1.0 |
0.0 |
8.1 |
|
3.0 |
0.0 |
0.0 |
40.2 |
|
3.0 |
0.0 |
0.1 |
40.3 |
|
4.5 |
0.0 |
0.0 |
89.9 |
Note: Pressure are in MPa, measured rate is on arbitrary units.
Are the data consistent with the proposed mechanism? State and justify any assumptions you have made.

