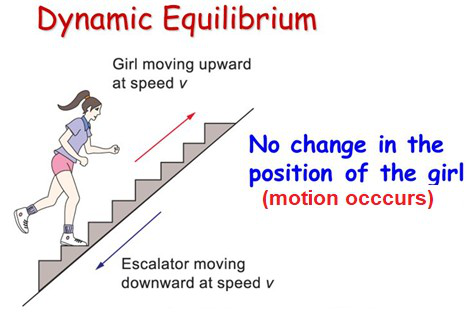
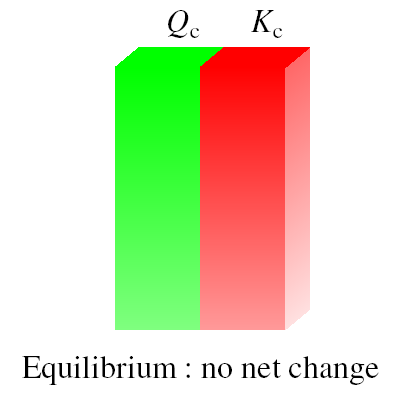
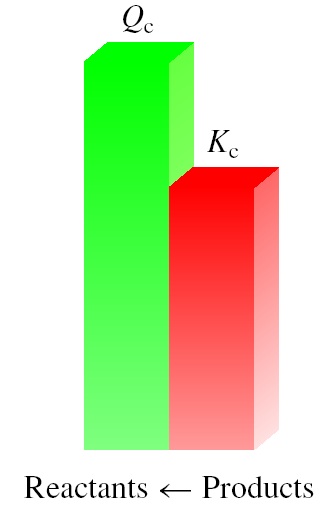
And the reaction proceeds the left reach equilibrium
• Nitrogen dioxide produces ditrogen tetraoxide on heating. 2NO2(g) N2O4(g)
N2O4(g) 2NO2(g)
equilibrium equilibrium
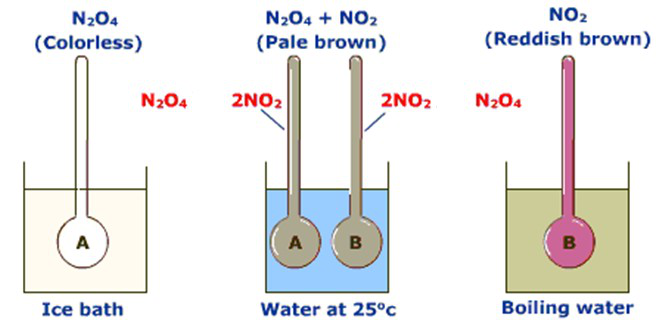
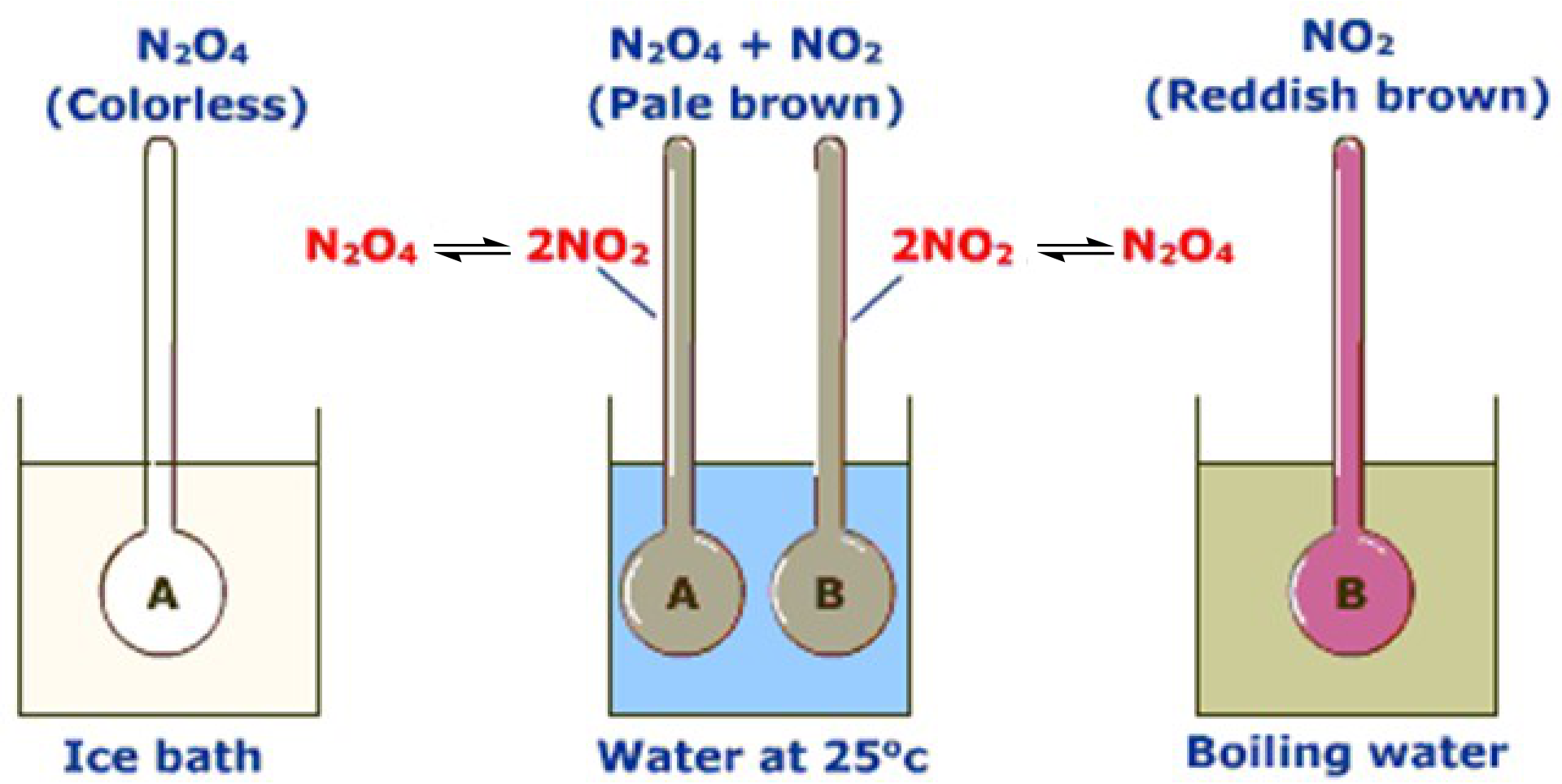
• Equilibrium can be attained starting with reactants or products.
equilibrium
• Both reactions occur until equilibrium is reached.
| • | Equilibrium can also be attained starting with both reactants and | 6 |
|---|---|---|
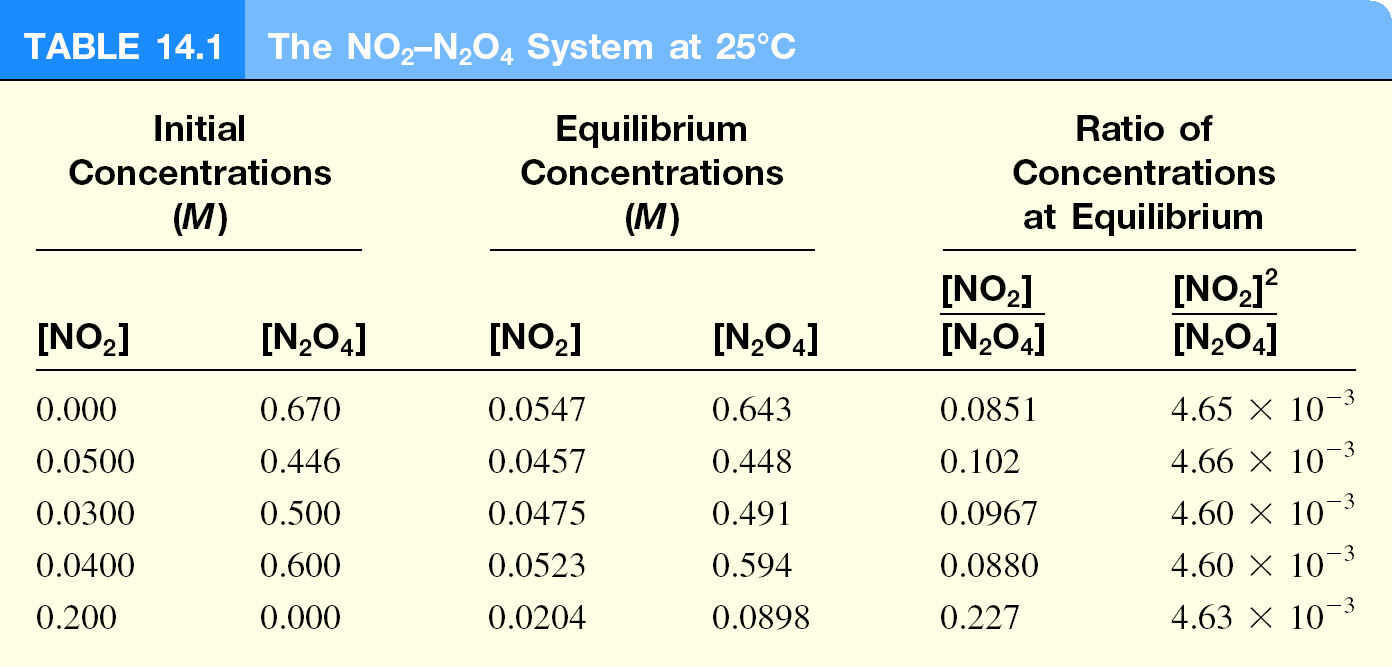
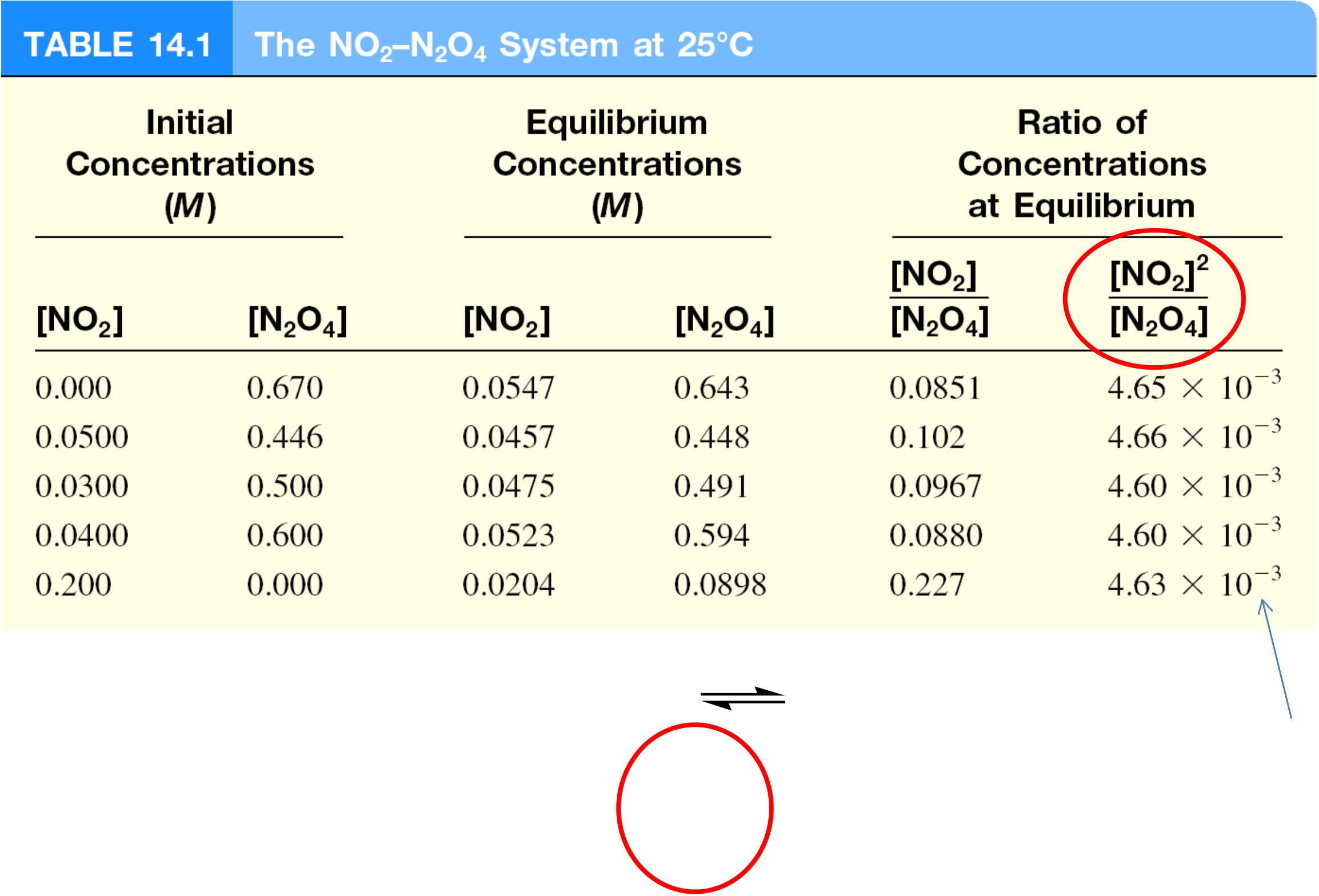
The Equilibrium Constant, Kc
Different initial concentrations of NO2 and N2O4 allowed to react until
chemical equilibrium was established. Equilibrium conc of each
measured.
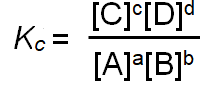
| equilibrium law |
|---|
[ ]refers to molar concentration at equilibrium
(i)N2(g) + 3H2(g) 2NH3(g)
(ii) 2SO3(g) 2SO2(g) + O2(g)
N2H4(g) + 6H2O2(g) 2NO2(g) + 8H2O(g)
d.
• Note the following:
for the equilibrium reaction
12
3. The following reactions have equilibrium values all measured at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion greatest completion).
• For the homogeneous equilibrium system N2O4(g) 2NO2(g),
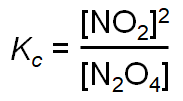
is used instead of concentration to calculate equilibrium constant.


n = change in the number of moles of gas
What is n for the reaction below?


• For the homogeneous equilibrium reaction
| Kc =′ | ||
|---|---|---|
Kc = [CH3COO-][H3O+]
[CH3COOH]• General practice not to include units for the equilibrium constant not to include pure liquids (and pure solids) in the equilibrium constant expression.
(a) HF(aq) + H2O(l) H3O+(aq) + F-(aq)
(b) 2NO(g) + O2(g) 2NO2(g)
Solution
(a) Because there are no gases present, KPdoes not apply, and we have only Kc.HF(aq) + H2O(l) H3O+(aq) + F-(aq)
(c) Because there are no gases present, KPdoes not apply, and we have only Kc.
CH3COOH(aq) + C2H5OH(aq ) CH3COOC2H5(aq) + H2O(l)
5.Kp is related to Kc by the equation Kp = Kc (RT)n. What is the
value of n for the reaction below?
| 19 |
|---|
Problem
The following equilibrium process has been studied at 230°C: 2NO(g) + O2(g) 2NO2(g)
In one experiment, the concentrations of the reacting species at equilibrium are found to be [NO] = 0.0542 M, [O2] = 0.127 M, and [NO2] = 15.5 M. Calculate the equilibrium constant (Kc) of the reaction at this temperature.Solution
Strategy
Write the equilibrium constant expression, Kp. What is the
unknown?
21
| 1.05 = | 2 | ) | |||||
|---|---|---|---|---|---|---|---|
| (0.875) |
|
||||||
| P | = | (1.05)(0.875) | |||||
| Cl | 2 | (0.463) | |||||
22
23
similar problem 14.17

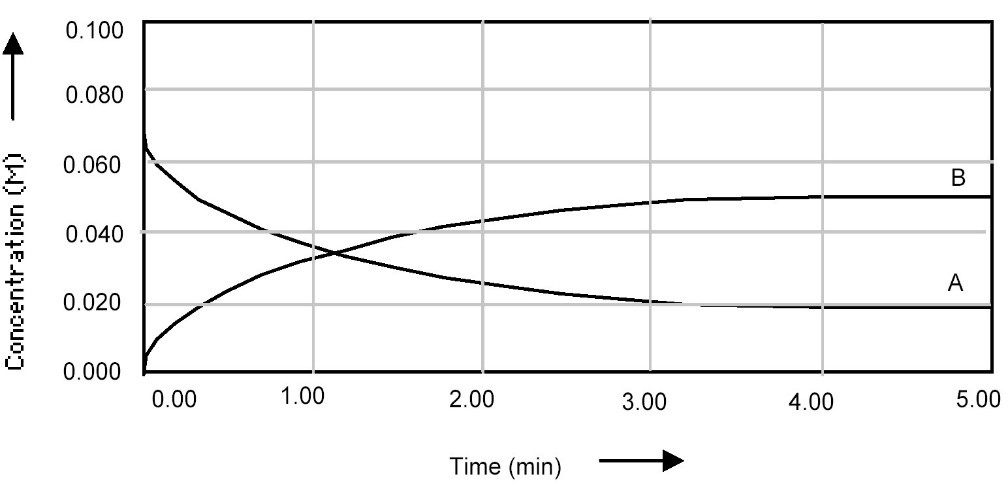


































7. Shown below is a concentration vs. time plot for the reaction A 2B. For this reaction, the value of the equilibrium constant is
A) Kc < 1
B) Kc = 0
C) Kc = 1
D) Kc > 1
was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a
4.0 L vessel. Calculate the equilibrium constant, Kc for this reaction.
• Heterogenous equilibrium applies to reactions in which reactants and products are in different phases.



Problem
Write the equilibrium constant expression Kc, and Kp if
applicable, for each of the following heterogeneous
systems:
28
Solution
K p = P NH 2 |
|---|
29
(c) We note that P4 is a solid and PCl3 is a liquid, so they do not appear in the equilibrium constant expression. Thus, Kc and Kp are given
| by | K | = | 1 | |||||
|---|---|---|---|---|---|---|---|---|
| c | K | p | = | P Cl 6 | 30 | |||
8. Write the equilibrium constant expression for the following reaction:

Problem
(b) We know that
Kp= Kc(0.0821 x T)ΔnIn this case, T = 800 + 273 = 1073 K and Δn = 1, so we substitute these values in the equation and obtain
Multiple Equilibria (Manipulating the Equilibrium Constant)
• For the reaction N2O4(g) 2NO2(g)
• For the reaction 2N2O4(g) 4NO2(g)
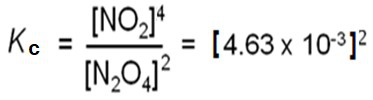
• Suppose two equilibrium equations are added to give a third
equilibrium equation. How does the equilibrium constant for the third equation relate to the other two?
(a) Write the equilibrium constant expression for each formulation.
(b) How are the equilibrium constants related to each other?
The following equilibrium constants have been determined for hydrosulfuric acid at 25oC.
H2S(aq) H+(aq) + HS(aq) Kc1 = 9.5 x 10-8 HS(aq) H+(aq) + S2(aq) Kc2 = 1.0 x 10-19
10. Given the two reactions shown with their equilibrium constants,
Writing Equilibrium Constant Expressions (Guidelines Summary)
1. The concentrations of the reacting species in the
condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm.
40
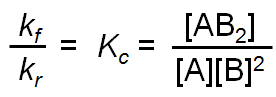



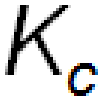
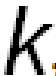
at equilibrium, ratef = rater
kf[A][B]2 = kr[AB2]
| • |
|---|
Predicting the Direction of a Reaction
For the reaction H2(g) + I2(g) 2HI(g), Kc = 54.3 at 430oC. Suppose the
following mixture is heated.
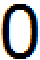
initial concentrations of the reactants and products into the
equilibrium constant (Kc) expression
• Since Qc is greater than Kc (54.3) then the numerator [HI]2 is too large.
The reaction below occurs to make HI smaller:
Strategy
Calculate Qc using initial concentrations and then compare Qc to Kc.
45
12. The system, 2H2O(g) + 2Cl2(g) 4HCl(g) + O2(g), has a value of 8.00 for Kp. Ini tially, the partial pressures of H2O(g) and Cl2(g) are set at 0.100 atm, while those of HCl(g) and O2(g) are set at 0.250 atm. Which statement below is true?
Calculating Equilibrium Concentrations
1. Set up an (R)ICE table.
2. Express the equilibrium concentrations of all species in terms of the
initial concentrations and a single unknown x, which represents the
4. Having solved for x, calculate the equilibrium concentrations of all
species.
Calculate the concentrations of H2, I2, and HI at equilibrium.
Strategy
Step 1: The stoichiometry of the reaction is 1 mol H2 reacting with 1 mol I2 to yield 2 mol HI. Let x be the depletion in concentration (mol/L) of H2 and I2 at equilibrium. It follows that the equilibrium concentration of HI must be 2x. We summarize the changes in concentrations as follows using a RICE (or ICE) table :
|
H2 + | I2 | 2HI | |
|---|---|---|---|---|
| 0.500 | 0.500 | |||
| - x | - x | + 2x | ||
|
|
(0.500 - x) | 2x |
| x | |
Step 3: At equilibrium, the concentrations are
[H2] = (0.500 - 0.393) M = 0.107 M
Strategy
The balanced equation shows that one mole of carbon monoxide will combine with one mole of water to form hydrogen and carbon dioxide. Let x be the depletion in the concentration of either CO or H2O at equilibrium. The equilibrium concentration of hydrogen must then also be equal to x. The changes are summarized as shown in the RICE/ICE table.
52
the reaction reaches equilibrium, 0.72 moles of PCl5 still remains. What is the
value of the equilibrium constant for this reaction? A) 0.62 B) 0.72
Equilibrium (M) 0.72
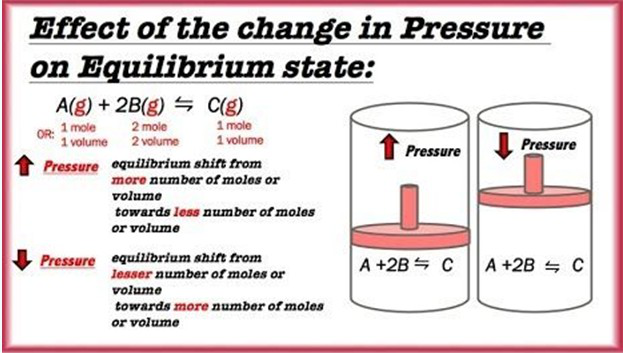
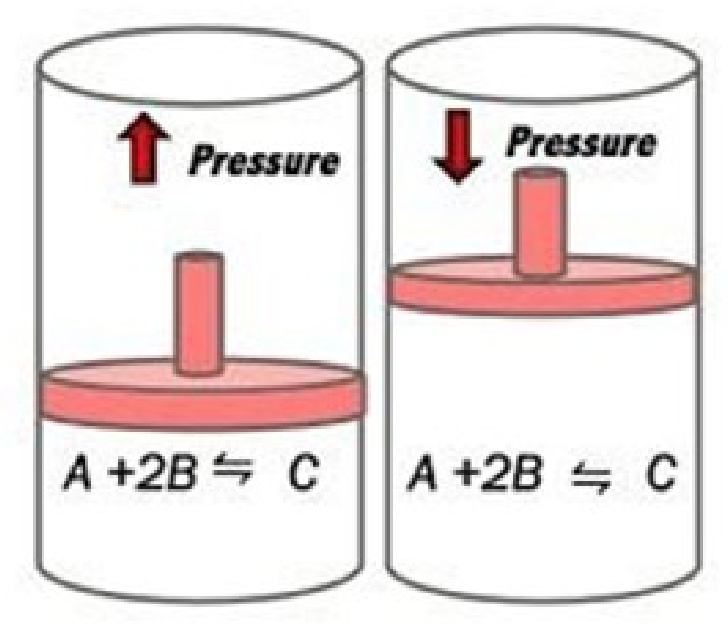
• Certain changes in experimental conditions such as temp, pressure, etc. can disturb the balance and change the amount of reactants and products.
• Le Chatelier’s principle predicts how these changes influence an equilibrium system: If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is reduced.
How can the equilibrium be restored if an external factor forces
the girl to slow down?
(ii) N2(g) is removed?



























































































































































































































































































































































































































































































































































































































































































































|
|
|
|---|---|---|
• As volume increases, pressure decreases [Boyle’s law].
PCl5(g) PCl3(g) + Cl2(g)



(c) H2(g) + CO2(g) H2O(g) + CO(g)
61
(ii) If T decreases, eq system will want to increase temp. Reaction shifts to the right forming more NH3.
increasing the reaction temperature would A. Shift the
equilibrium to the right and increase the value of the equilibrium constant K B. Shift the equilibrium to the left and increase the value of the equilibrium constant K C. Shift the equilibrium to the right and decrease the value of the equilibrium constant K D. Shift the equilibrium to the left and decrease the value of the equilibrium constant K
| 16. For the reaction at equilibrium: 3Fe(s) + 4H2O(g) |  |
|
|---|---|---|
B. 2NO(g) + Cl2(g)
2NOCl(g)
C. 2SO3(g)
2SO2(g) + O2(g)
• Catalyst lowers Ea for both forward and reverse reactions.
ΔH° = 38.5 kJ/mol
Predict the changes in the equilibrium if
(a) the reacting mixture is heated at constant volume
similar problem 14.58


(5) Add a catalyst
19. The pink and blue species below form a
violet-colored mixture at equilibrium:
[Co(H2O)6]2+(aq) + 4 Cl-(aq) [CoCl4]2-(aq) +
6 H2O(l)
(pink) (blue)
| 67 |
|---|
CO(g) + 3H2(g) CH4(g) + H2O(g) at equilibrium?
(1) H2O will be consumed. (2) More CH4 and H2O will be produced. (3) Kp will decrease. (4) More CO will be produced. (5) No change will occur.
(2) Increasing the temperature shifts the equilibrium to the right.
(3) A catalyst speeds up the approach to equilibrium and shifts the position of equilibrium to the right.
22. When the substances in the equation below are at equilibrium, at pressure P and
temperature T, the equilibrium can be shifted to favor the products by CuO(s) + H2(g) H2O(g) + Cu(s) = –2.0 kJ/mol
CO(g) + Cl2(g) COCl2(g)
(1) 0.81 atm (2) 2.12 atm (3) 1.31 atm (4) +0.50 atm (5) -0.50 atm
69